Content:
1. Introduction
2. Mitochondrial electron transport chain
_
Introduction
Oxidation and the role of oxygen
Oxidation is defined in chemistry as the removal of electrons or decreasing of the oxidation number of an element. Since these removed electrons must end up on some other element oxidation of one compound is always accompanied by the reduction of another. The word ‘oxidation’ itself comes etymologically from ‘oxide’, a compound containing oxygen. As we shall see shortly, oxygen is in fact not required for oxidation, as defined above, to take place.
Oxygen is an excellent oxidising agent; it loves receiving electrons. The affinity of an element for electrons is expressed by the element’s electronegativity. Oxygen has one of the highest electronegativities of all elements; in fact it is second only to fluorine. This means that the transfer of electrons onto oxygen including the formation of bonds with oxygen is thermodynamically favourable, i.e. it releases energy. The only reason we don’t spontaneously combust in our 20 % oxygen atmosphere is the kinetic barrier created by the spin constraints of triplet dioxygen (see Subchapter 2/4).
Organisms living in a highly oxidising environment (such as ours) quickly found a way to harness the energy of available from transferring electrons from less electronegative elements to oxygen. The often used metaphor for the oxidative metabolism is the “burning of nutrients.” When we light up a campfire organic polymers in wood are oxidised by oxygen to carbon dioxide and water and we enjoy the energy released by this redox reaction as the soothing heat and flickering light that make campfires so much fun. In an analogous (but quite dissimilar) way our cells oxidise organic chemicals which we receive in diet to CO2 and H2O while consuming oxygen and producing energy. How exactly do they do it?
Unlike in a camp fire, in our cells the transfer of electrons from an organic molecule to oxygen is divided into many steps. Also most of the energy gained by these reactions is not released as heat and light but stored as chemical potential. For didactic purposes we can divide oxidative metabolism into two phases:
1) Substrate oxidation with reduction of coenzymes
2) Reoxidation of reduced coenzymes by oxygen.
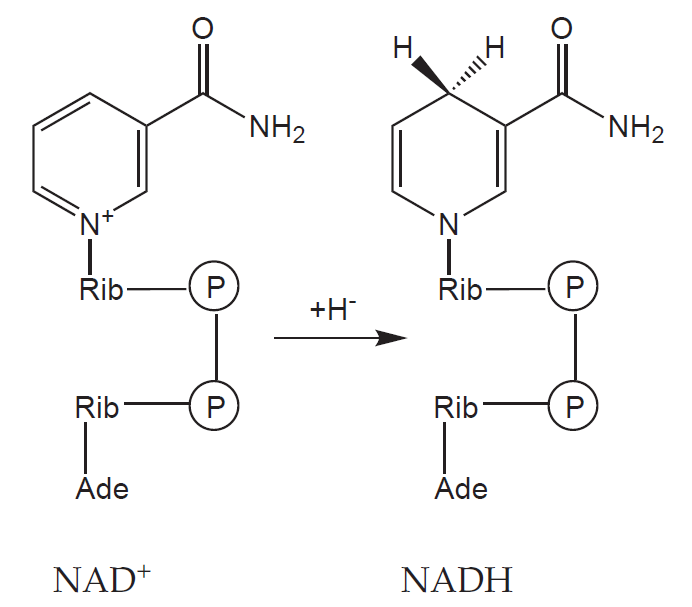
Let us take glucose as an example. The six carbon molecule (C6H12O6) is gradually oxidised in glycolysis to yield two molecules of acetic acid (in the form of acetyl coenzyme A, acetyl-CoA) and two molecules of CO2. In the process four molecules of the redox coenzyme nicotinamide adenine dinucleotide (NAD+) are reduced to nadh, each receiving two electrons (often denoted as an hydride anion, H–).
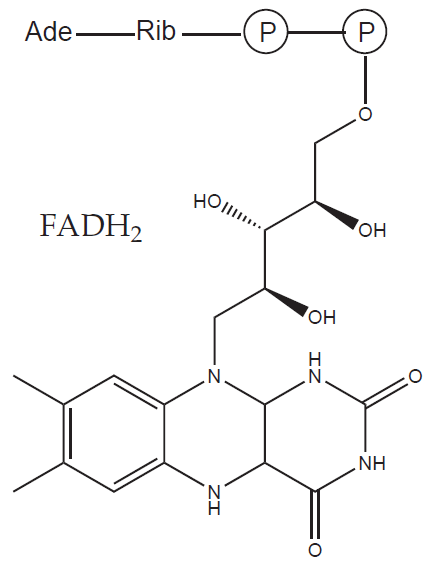
Each acetyl-CoA goes on into the citric acid cycle and is further oxidised to two molecules of CO2 and four reduced coenzymes (three NADH and one flavin adenine dinucleotide, FADH2).
Overall, the oxidation of a glucose molecule yields 12 reduced coenzymes and (as expected from the elemental composition) six molecules of CO2. Please note that no molecular oxygen (O2) is needed in this process – the missing oxygen atoms are provided by water. Water contains oxygen already in a reduced form (O-II) so in these reactions oxygen is not part of the electron transfer.
The reduced coenzymes must be reoxidised in order to keep this metabolic pathway going. Moreover, as we mentioned above, the reduction of oxygen promises to release a substantial amount of energy. The reoxidation takes place in mitochondria.
_
Mitochondrial electron transport chain
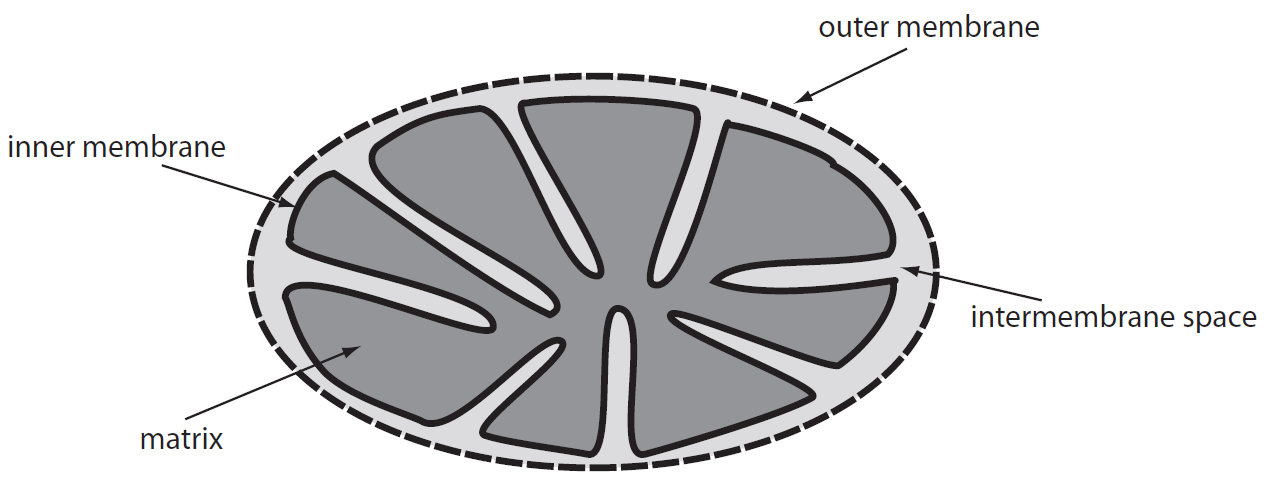
Mitochondria
Mitochondria are complex organelles with two membranes (outer and inner), which enclose the intermembrane space and the mitochondrial matrix respectively. While the outer membrane is freely permeable for small molecules (< 5000 Da), the inner membrane is almost completely impermeable for polar molecules (with the exception of a select few which have dedicated transporters). The mitochondrial matrix is a thick protein gel containing enzymes catalysing reactions in crucial metabolic pathways such as the citric acid cycle or beta-oxidation of fatty acids.
Electron transport chain
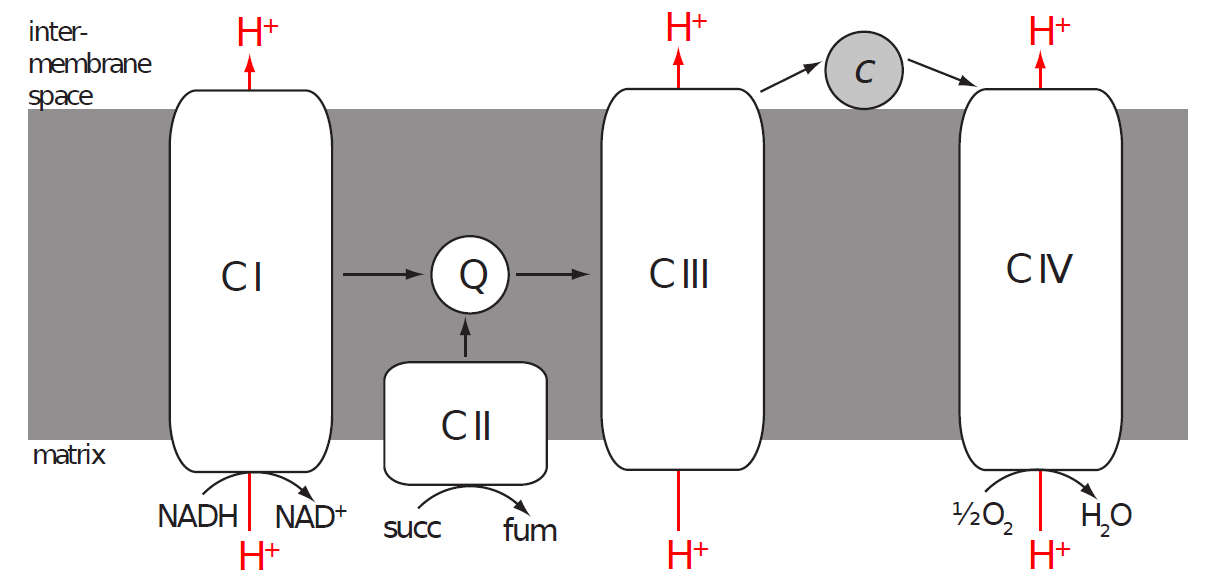
Reduced coenzymes arriving from the cytoplasm (via a special shuttle system) and from reactions occurring in the matrix are reoxidised in the inner mitochondrial membrane by a collection of enzymes called the electron transport chain (ETC, or respiratory chain). The mitochondrial ETC is composed of four enzyme complexes named Complex I-IV.
Electrons from NADH enter into Complex I and are transferred via Complex III and IV onto oxygen. Electrons from FADH2 are extracted by Complex II and other enzymes (bypassing Complex I) and then follow the same path.
Complex I (NADH dehydrogenase or NADH:ubiquinone oxidoreductase)
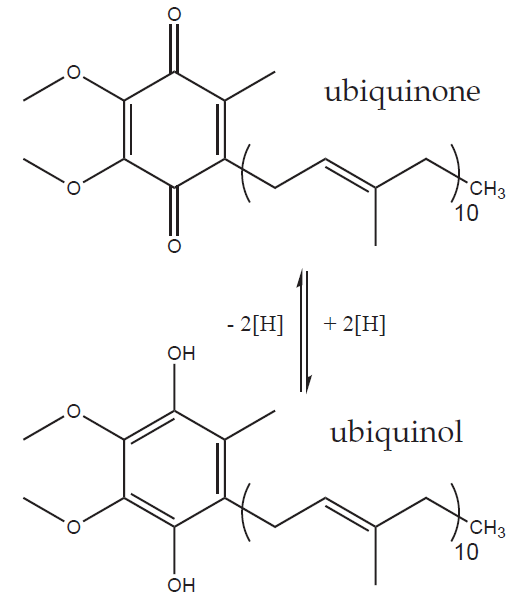
Complex I catalyses the oxidation of NADH to NAD+ and the transfer of two electrons onto coenzyme Q (CoQ) or ubiquinone. The precise structure of the mitochondrial Complex I is still unknown but we know that it contains over 40 subunits (450 000 Da), one molecule of flavin mononucleotide (FMN) and several atoms of iron complexed into iron-sulphur clusters (FeS). The electrons released from nadh bind to fmn and then cascade one at a time from one FeS cluster to the next until they arrive to ubiquinone and reduce it to ubiquinol.
Ubiquinol (CoQH2 or UQH2) is the reduced form of ubiquinone (CoQ or UQ). Coenzyme Q (an umbrella term for both redox forms) is an extremely hydrophobic molecule thanks to its very long isoprenoid side chain (ten five-carbon isoprenoid units in the mammalian CoQ10), which locks it safely in the non-polar core of the inner mitochondrial membrane. There CoQ acts as a mobile electron carrier from Complexes I and II (and some other enzymes) to Complex III.
Complex II
Complex ii catalyses the oxidation of succinate to fumarate and as such is an integral part of the citric acid cycle (succinate dehydrogenase or succinate:ubiquinone oxidoreductase). The electrons gained by this oxidation are first transferred to FAD bound in the enzyme and then via a chain of three FeS clusters and cytochrome b onto ubiquinone.
Complex III (cytochrome c reductase or ubiquinol:cytochrome c oxidoreductase)
Complex III accepts electrons from the reduced CoQ pool and transfers them (via two cytochromes and an FeS cluster) onto another mobile electron carrier, cytochrome c. Cytochrome c is a small hemoprotein attached to the external surface of the inner mitochondrial membrane, i.e. in the intermembrane space. Unlike previously discussed coenzymes cytochrome c can only carry one electron per molecule (reducing its haem iron from ferric to ferrous). Electrons from ubiquinol are therefore transferred one by one in a complex process called the Q-cycle.
Complex IV (cytochrome c oxidase or cytochrome c:dioxygen oxidoreductase)
Complex IV is the final member of the ETC, which takes electrons from reduced cytochrome c and via two cytochromes a and three copper atoms and deposits them on the final electron acceptor, oxygen.
Redox potential
In order for the mitochondrial ETC to function as described above there must be a force pushing the electrons from NADH to O2 along the chain. In the case of burning wood we talked about the electronegativity of elemental oxygen. A related measure of affinity for electrons is the redox potential.
In Subchapter 2/4 we discussed electrode potentials created by dipping a bar of pure metal into a solution of its ions (i.e. its oxidised form). We noted that if we separate the two half-reactions present in every redox reaction – reduction half-reaction and oxidation half-reaction – we can define standard electrode potentials for these reactions occurring under standard conditions. Depending on the direction of these reactions we often call such potentials standard oxidation or standard reduction potentials. The umbrella term is redox potential, which is usually in the direction of reduction.
The reason why electrons flow from NADH to oxygen through the ETC complexes and mobile electron carriers in the right order is that all the individual “stops” along the way have increasing redox (more specifically reducing) potentials. This means that as we go along the chain things are becoming easier and easier to reduce. Oxygen at the end is by far the easiest to reduce – that’s why it’s such a great oxidising agent!
Where did the energy go?
When we started off discussing oxidative metabolism we mentioned that the advantage of using oxygen as the final electron acceptor lay in the amount of energy that can be made available. So far we have followed electrons on their way to being united with their Final Acceptor but no mention has yet been made of how our cells take advantage of it.
The large increase in the reducing potential between NADH (or FADH2) and oxygen and the corresponding difference in free energy (ΔG) is indeed not wasted. It is used to pump protons (H+) from the mitochondrial matrix into the intermembrane space.
The flow of electrons through Complexes I, III and IV is coupled with pumping a certain number of protons per electron pair; Complex II does not pump anything. Since the inner mitochondrial membrane is highly impermeable for protons a proton potential builds across it – there are more protons in the intermembrane space than in the matrix. Higher proton concentration means lower pH and a positive electrical potential. The intermembrane space in a respiring mitochondrion is therefore more acidic and more positive than the matrix. The mitochondrial membrane potential is usually expressed as voltage.
As gravitational potential energy of tons of water stored in a reservoir can be used in a hydroelectric power plant to make electricity, our mitochondria use the energy stored in the proton gradient across the inner membrane to make a different kind of energy – chemical energy stored in adenosine-5’-triphosphate (ATP).
The synthesis of ATP is catalysed by an enzyme called F1.FO-ATP syntáza. The FO subunit forms a channel in the inner mitochondrial membrane which allows protons from the intermembrane space to flow back into the matrix. This flow of protons along the electrochemical gradient is used to rotate part of the enzyme in the same way as water flowing through a dam makes a turbine rotate. The rotation is then transferred onto the central stalk of the F1 subunit, which pushes on the outer subunits held stationary by the peripheral stalk and makes them phosphorylate adenosine-5’-diphosphate (ADP) to ATD. Since the F1 subunit has three sites for ATP synthesis a complete turn of the enzyme makes three molecules of ATP.
The newly synthesised molecules of ATP are then transported out of the matrix into the cytoplasm via a special transporter (adenine nucleotide translocator, ANT) in exchange for ADP.
Stoichiometry
All the processes described above are primarily about getting energy from a substrate in order to do some useful work. It is therefore understandable that we should like to know how much useful energy our mitochondria can extract e.g. from a molecule of glucose or palmitic acid. While this topic is usually treated rather summarily in many textbooks it is far from trivial.
We have discussed above the number of electrons removed (as reduced coenzymes) in the metabolism of glucose; similar numbers can be calculated for other substrate. The question of mitochondrial stoichiometry can thus be formulated as follows: how many molecules of ATP can be synthesised by the ETC per certain number of electrons transported. This can be further divided into two sub-questions:
1) How many protons are transported across the membrane per one electron pair?
2) How many protons must be translocated back into the matrix in order to make one molecule of ATP?
The current consensus answer to question 1) is 10 protons per two electrons. Complexes III and IV transport together six protons and Complex I probably transports four protons per electron pair. Since the pumping mechanism on a molecular level is still only partially understood, these numbers could change in the future.
Answering question b) is even more tricky. Based on current theoretical models of function of F1.FO-ATP synthase the H+/ATP ratio appears to be 4.33, in other words 13 protons are translocated to make three molecules of ATP (10 protons translocated by F1.FO-ATP synthase, additional three are used to import ADP and phosphate and export ATP via ANT). A definitive answer to these question lies still in the future.
At the same time it is important to realise that these numbers are the maximum values attainable under perfect conditions when all components of ETC work flawlessly and the inner mitochondrial membrane is absolutely impermeable to protons. This, however, is usually not the case.
Uncoupling
The mechanism of energy transduction between the ETC and the synthesis of ATP can be uncoupled by allowing protons to flow from the intermembrane space back into the matrix. Such ineffectual dissipation of the proton gradient converts the energy stored there into the least useful kind of energy: heat.
Despite heat being generally far less useful than for example ATP, there is one situation in which being able to produce heat can be life saving -namely when it’s freezing cold and you just realised you left your coat, sweater, thermal underwear… in fact all your clothes, at home. A well known heat producing mechanism we (and other mammals) use is shivering, which creates heat through inefficiencies in the energy transduction of muscle contraction. Newborn babies and a host of animals have another method – mitochondrial uncoupling.
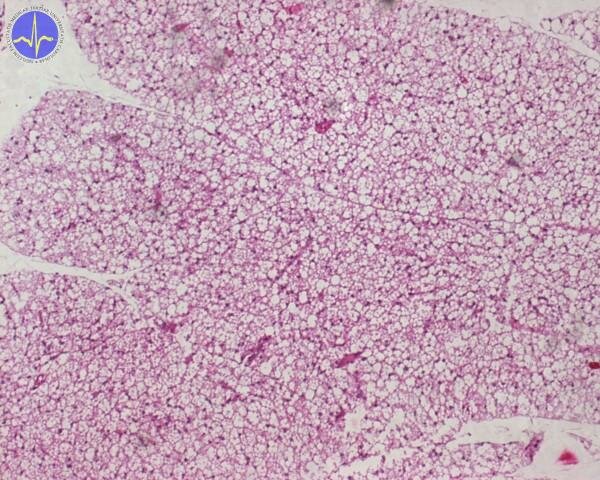
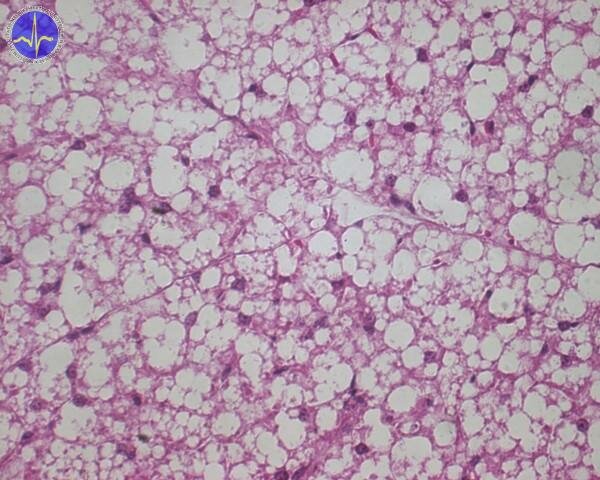
Mitochondrial uncoupling for the production of heat takes place in a special tissue called the brown adipose tissue. Brown fat (in contrast to normal white fat) is brown because it’s full of mitochondria.
Brown adipose tissue 2x – cell contain more adipous droplets of various size.
While white fat is there for the storage of energy, the role of brown fat (in those who have it) is to waste it. The mitochondria in brown fat contain a special protein, which forms a channel in the inner mitochondrial membrane for the translocation of protons – uncoupling protein-1 (UCP-1). When the brown adipose tissue is activated by noradrenaline (via β3-adrenergic receptor) it hydrolyses its triglycerides and the resulting free fatty acids both provide energy for the ETC and activate UCP-1.
There are other subtypes of UCP (UCP-2 – UCP-5) expressed in other tissues but their physiological function is still unclear.
_
Subchapter Author: Jan Trnka