Content:
1. Importance of compartmentalization
2. Biological membranes
3. Intracellular transport
4. Compartmentalization of metabolic pathways
_
Importance of compartmentalization
All reactions occurring in cells take place in certain space – compartment, which is separated from other compartments by means of semipermeable membranes. They help to separate even chemically quite heterogeneous environments and so to optimise the course of chemical reactions.
Enzymes catalysing individual reactions often have different temperature and pH optimums and if there was only one cellular compartment a portion of enzymes would probably not function or them-catalysed reactions would not be sufficiently efficient. By dividing the cellular space, optimal conditions for individual enzymatically catalysed reactions are created.
At the same time, cell also protects itself against the activity of lytic enzymes. For example, sealing the cellular digestion in lysosomes prevents an unwanted auto-digestion of other organelles within cell. A common processes that accompany the disruption of some of the compartments (like spilling the content of lysosomes or mitochondria) are necrosis or activation of apoptosis (the process of programmed cell death).
Compartmentalization affects the regulation of metabolic pathways as well, making them more accurate and targeted and less interfering with each other. It is sometimes possible to regulate the course of the reaction at the point of entry of particular substrate into the compartment (transport across the membrane, often mediated by transport mechanisms).
Despite its advantages, compartmentalization at the same time puts greater demand on the energy consumption. It arises from a frequent need to use ATP-dependent transporters, transporting substances across membranes against the concentration gradient and thus creating different environments in different compartments.
_
Biological membranes
One of the necessary conditions for the emergence of live was the separation of cellular environment from the outside (external) environment. Resulting from this, there was a need for selective transport mechanisms between both spaces and later a need for intercellular communication.
Cytoplasmic membrane creates the border with the extracellular compartment and membranes of a similar composition separate other compartments inside the cell.
Architecture
The width of a cell membrane is approximately 6-10 nm. The core of its architecture is made of phospholipid bilayer with embedded proteins and cholesterol molecules. The latter two can bind various saccharides and so form glycolipids and glycoproteins. This basic structure is, in the case of membranes of different organelles, modified to a certain degree, thus affecting the physico-chemical properties of the membrane (especially its permeability), which are in close connection to the function and course of the biochemical processes in the organelle.
A good example is the myelin sheath of a neuron, which has a ratio of proteins to lipids 19 % to 81 % and thus has excellent insulating properties. On the other hand, the inner mitochondrial membrane has the ration reversed in a favor of proteins (76 % to 24 %) and that is the reason for its relative impermeability (even for substances that pass through standard membranes).
Molecules of phospholipids consist of two physically distinct parts:
1) Polar (hydrophilic) part
The polar part is made up of a phosphate group with other optionally attached groups – this part of the molecule faces the aqueous medium (or other polar solvent).
2) Non-polar (hydrophobic) part
The non-polar part consists of fatty acid chains turned against each other and thus forming the hydrophobic core of the membrane. The hydrophobic interactions of lipid molecules are responsible for their tendency to aggregate and form membranes.
Phospholipid molecule, containing polar as well as non-polar part, belongs to the group of amphipathic molecules.
Historical correlation:
The currently accepted model of the structure of biological membranes was created in year 1972 by S.J. Singer and G.L. Nicols. According to this, so-called fluid-mosaic model, we can consider the biological membrane as a 2-dimensional liquid in which lipid and protein molecules diffuse more or less easily.
The diffusion rate of phospholipids is much faster than that of other membrane components. Places of membrane with higher content of proteins and cholesterol therefore have lower lateral diffusion rate and stabilise membrane (this applies especially to cholesterol). On the other hand, parts of membrane containing mostly lipids have the ability to flip to the opposite side (through so-called flip-flop mechanism).
The fluidity of membrane depends mostly on:
1) The temperature: the higher the temperature the more mobile the membrane is (sol phase), at lower temperatures it is stiffer (gel phase).
2) The proportion of unsaturated FA: the higher the content of unsaturated FA, the more mobile the phase is.
Proteins form a basic component of cellular membranes. According to their localisation within the membrane, they can be divided into peripheral and integral:
1) Peripheral proteins
Peripheral proteins do not penetrate to the hydrophobic core of the membrane; they only bind to its surface area (on extra- or intracellular side) and thereby can be separated from the membrane without damaging it. The main binding interactions include electrostatic forces and hydrogen bonds.
2) Integral proteins
Integral proteins permeate the membrane, either through its full thickness (transmembrane proteins) or only up to a certain depth. Separation of these proteins is associated with the disruption of membrane integrity.
Membrane proteins perform various functions, for example receptor, enzymatic or transport.
Cholesterol makes up approximately ¼ of all membrane lipids. Its molecule, similarly to the molecule of phospholipids, has amphipathic character (due to the presence of OH- group attached to the 3rd carbon atom). The main function of cholesterol is to stabilise the membrane and lower its fluidity.
Properties
Permeability, expressing the rate of passive diffusion of molecules through the membrane, follows the Fick’s law of diffusion and depends on:
1) The size and the polarity of diffusing molecules
2) The concentration gradient
3) The thickness of a membrane
4) The surface area of a membrane
1) The size and polarity of diffusing molecules
In general, small and non-polar molecules pass through the membrane quite easily while larger and polar ones usually need transporters or channels.
2) The concentration gradient
The higher the concentration of a substance on one side of the membrane, the higher its tendency to pass to the other side. This rule applies to other gradients as well – electrochemical (given by the difference of charges on both sides) or osmotic (given by the difference of osmotically active particles on both sides of the membrane).
3) The thickness of a membrane
Substances pass more slowly through a membrane that is thicker.
4) The surface area of a membrane
Larger quantity of substance diffuses through the membrane per unit of time if the membrane has larger surface area.
Other properties of membranes are: the degree of thermal and electrical insulation, electric charge (the total charge of a cell membrane is negative – primarily due to the presence of negative sialic acids residues attached to the glycoproteins and glycolipids) and the ability of selective transport.
Selective transport
Selective transport can be divided to:
1) Passive
2) Active
3) Transport of macromolecules
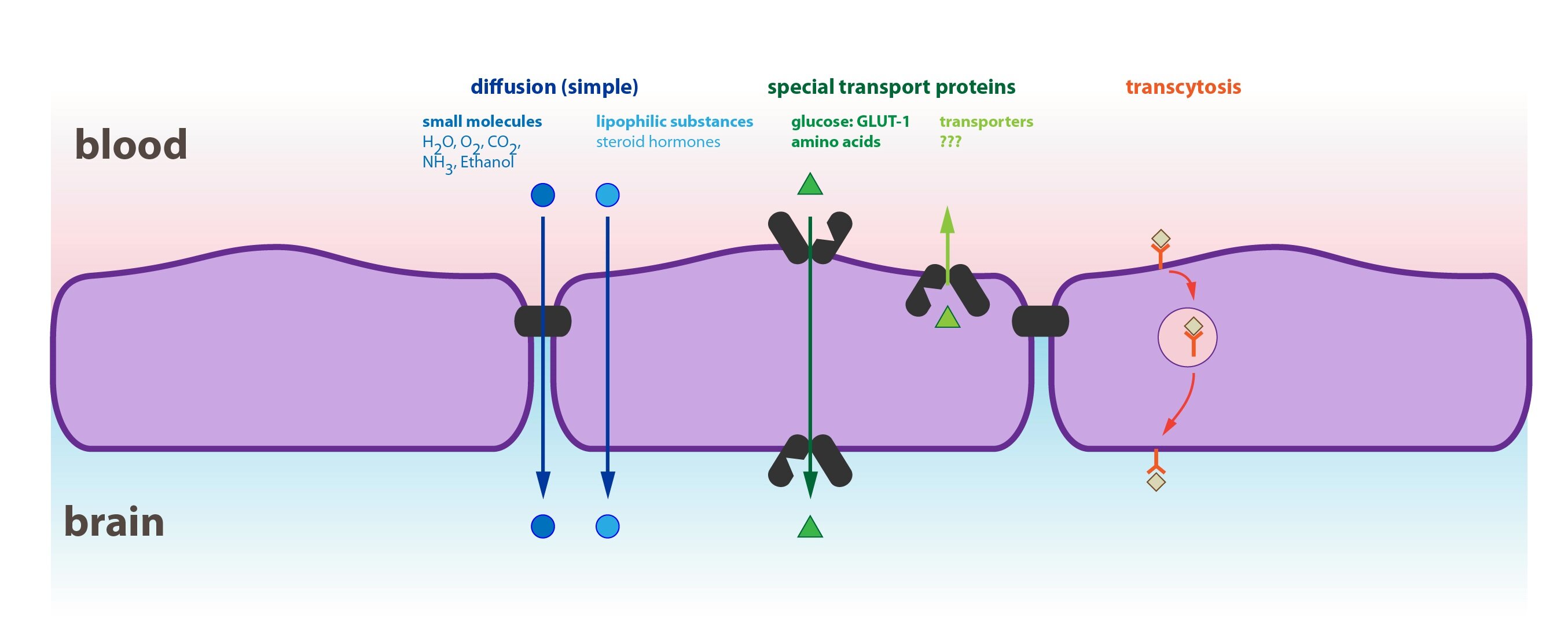
This diagram shows an example of transport processes through the blood-brain barrier (barrier between the blood and nervous tissue):
1) Passive transport
Passive transport takes place without energy consumption, as it is based on the physical principle of diffusion (driven by the concentration gradient of a substance on both sides of the membrane). Without the existence of such gradient, the passive transport halts. There are two basic types of passive transport:
a) Simple diffusion
b) Facilitated diffusion
a) Simple diffusion
Simple diffusion is a transfer of substances through a membrane without the help of transport proteins. Substances must pass through the hydrophobic core of the membrane, which explains why is this type of transport mechanism typical for:
1. Small non-polar molecule: like gases (CO2, O2, …)
2. Small polar molecule: water, urea
3. Larger non-polar molecules: FA, cholesterol or fat-soluble vitamins
Hydrophilic and larger molecules (mostly with Mr over 200) pass through the membrane by simple diffusion only very slowly or not at all. Transport of ions, whose molecules are relatively small, is difficult due to the presence of a large hydration shell consisting of water molecules.
b) Facilitated diffusion
Facilitated diffusion is a type of passive transport assisted by transport proteins that non-covalently bind the molecule and transport it to the other side of the membrane. It is faster than the simple diffusion and can be accompanied by a transport of other substance in the opposite direction (so-called antiport, for example ATP with ADP or Cl–with HCO3–). Another possibility is the transport with the help of channel protein that penetrates the whole width of the membrane. The transfer of a molecule is associated with the change of the channel’s conformation. Some channels are controlled through the changes in membrane electric potential (voltage-gated channels).
There is a difference between the kinetics of the simple and facilitated diffusion. In the case of simple diffusion, increasing the concentration of transferred substance leads to the linear increase in the rate of diffusion. Transport proteins involved in the facilitated diffusion, on the other hand, have limited capacity (given by their total number in the membrane) and when the concentration reaches high values, the rate of the diffusion slows down until it reaches its maximum speed (where the transport capacity is fully saturated).
Among the most important examples of facilitated diffusion are glucose GLUT transporters. Intracellular transformation of glucose to glucose-6-phosphate and its subsequent usage ensures the existence of continuous concentration gradient. Overall, there exist seven types of GLUT transporters of which the most important are:
1. GLUT 1 and 3, serving to maintain the basal glucose uptake by tissues, whose metabolism is dependent on glucose (brain, erythrocytes, kidneys or placenta).
2. GLUT 2, localised on the membrane of pancreatic β-cells and hepatocytes. It also enables the transfer of glucose form absorptive epithelia (e.g. the epithelia of proximal convoluted tubule of kidney, intestinal epithelia or enterocytes) to the blood.
3. GLUT 4 is the glucose transporter of so-called insulin-dependent tissues (skeletal muscle, myocardium and adipose tissue). Its presence on the membrane of these tissues is subject to the effect of insulin. This mainly occurs after the meal when are the above-mentioned tissues responsible of up to 80% of glucose metabolism. Between the meals, on the contrary, they do not absorb glucose and save it for the tissues that are dependent upon it.
2) Active transport
Active transport can take place against the concentration and electrochemical gradient as well. It is possible because the transport is coupled with the ATP hydrolysis (ATP → ADP a Pi) and the energy released is used in the process of transport. We recognize two basic types of this transport:
a) Primary
b) Secondary
a) Primary active transport
Energy of ATP is used directly in the process of transportation of a particular substance. Examples are Na+/K+-ATPase, H+/K+-ATPase or Ca2+-ATPase.
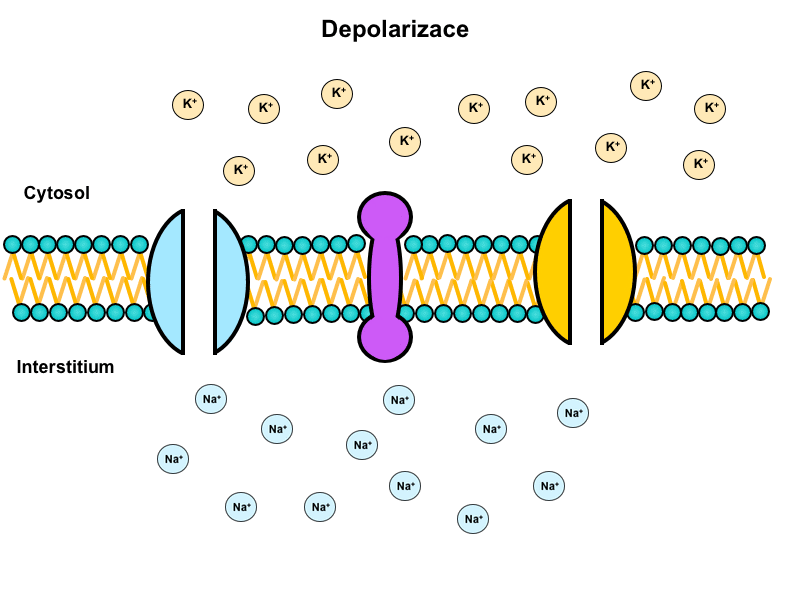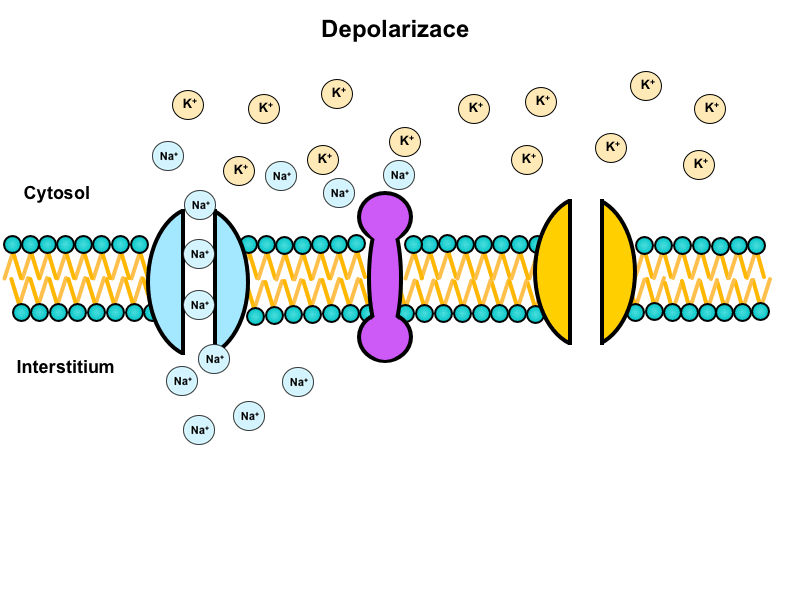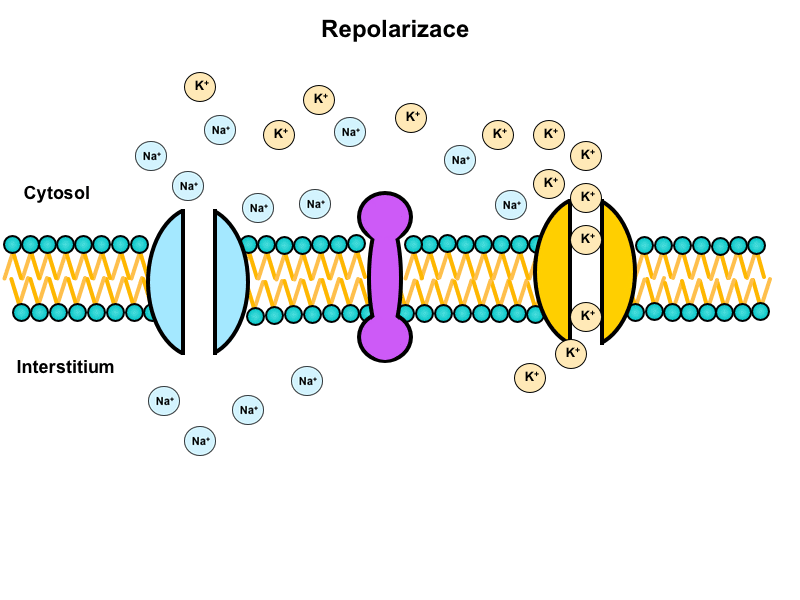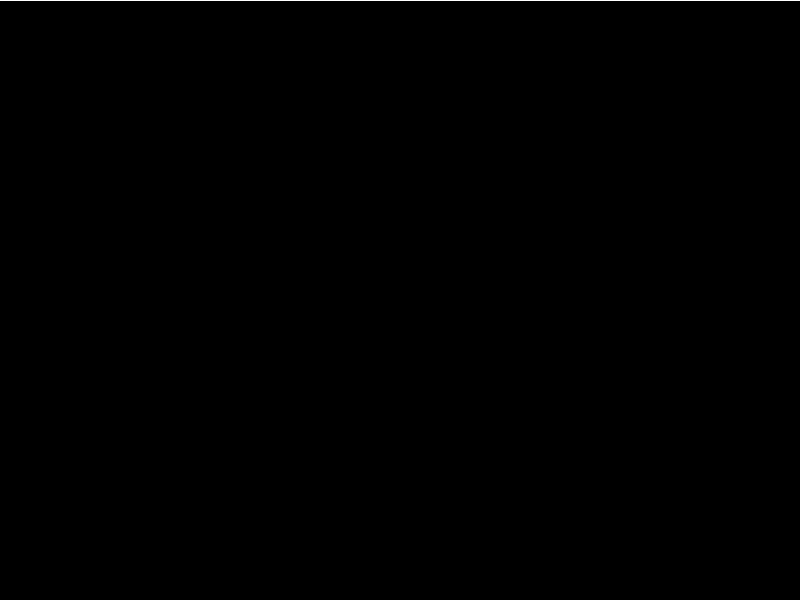
Na+/K+-ATPase is a tetramer made up of two alpha and two beta subunits. Alpha subunits are transmembrane, their intracellular domains have a binding site for Na+ and extracellular domains for K+. Beta subunits are glycolysed (as opposed to alpha) and do not penetrate through the whole membrane. Their oligosaccharide chains are facing the extracellular space. There exist two distinct conformational states of the enzyme, depending on whether it is phosphorylated or not. Na+/K+-ATPase serves as an antiport and in return for the energy released from the ATP it transports 3 Na+ cations out of the cell in exchange for 2 K+ cations. The result is an uneven distribution of ions on the membrane that forms the basis for the resting membrane potential. Na+/K+-ATPase is ubiquitous – it probably present in all human cells.
Animation shows function of this transporter (located in the center) during the action potential:
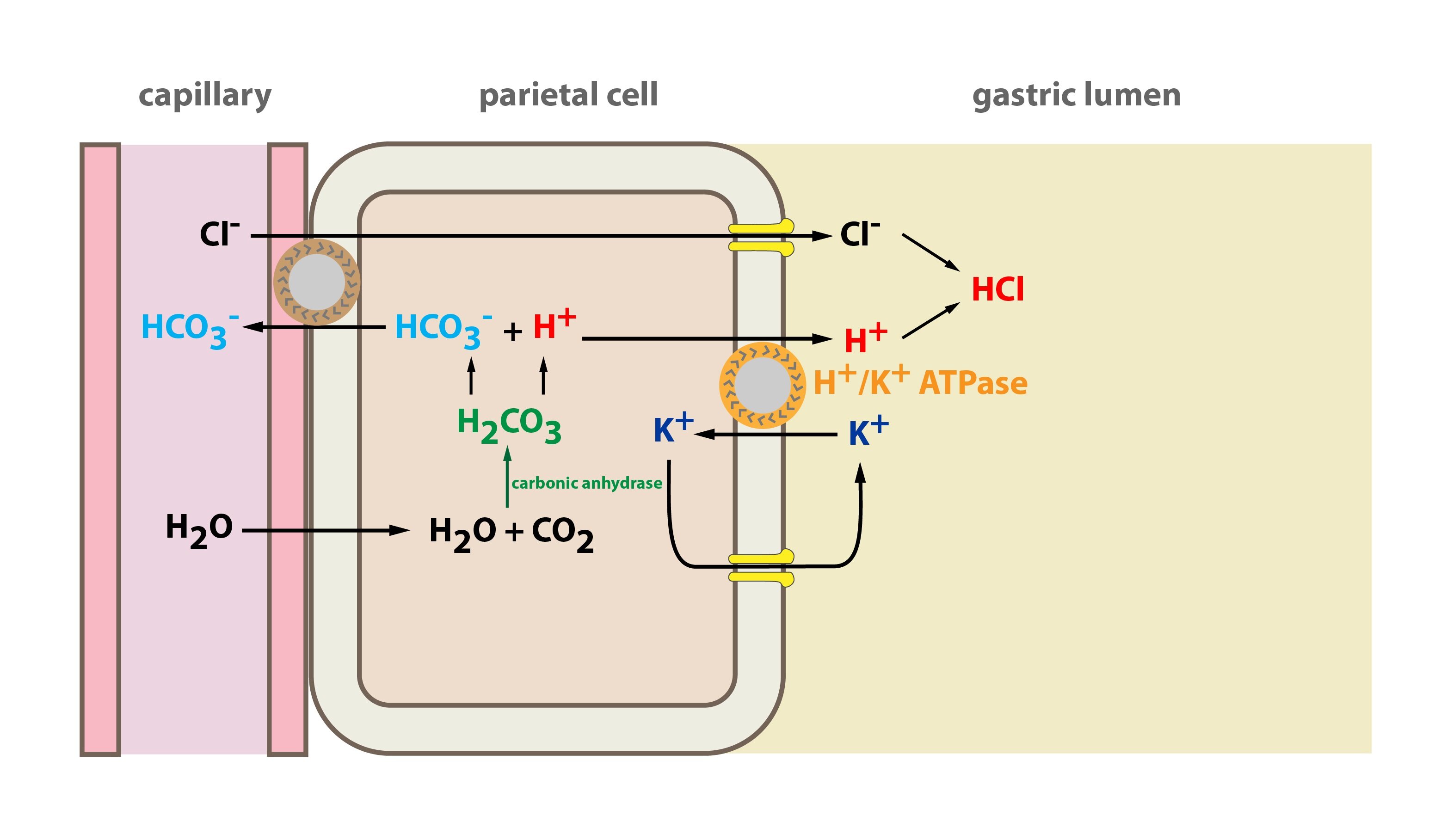
H+/K+-ATPase is an antiport operating similar to Na+/K+-ATPase. It can be found in the parietal cells of the stomach (where it produces the gastric juices) and in the cells of the proximal convoluted tubule of kidney. It transports one H+ out of the cell in exchange for one K+ ion.
Ca2+-ATPase, a calcium pump, is mostly localized in muscle and nerve cells. It actively pumps calcium ions out of the cytoplasm, either into the sarcoplasmic reticulum or extracellularly, thus lowering the Ca2+ concentration back to the previous level (for example before the contraction of a muscle cell).
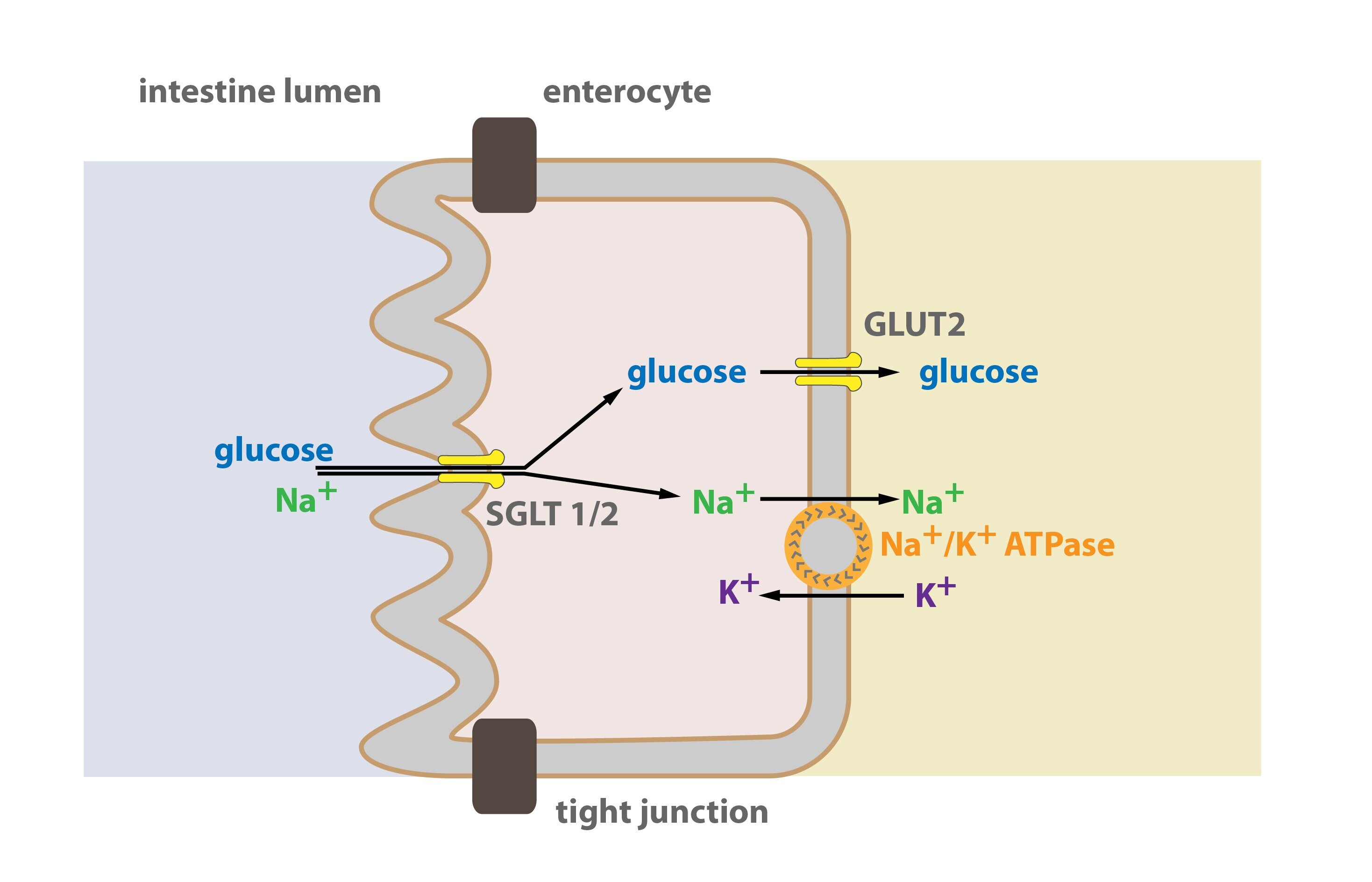
b) Secondary active transport (or cotransport)
In this case of active transport, the energy released from ATP is not directly used to transport the particular molecule (like glucose). Instead, it is used to transport other substances (e.g. sodium cation), which causes the formation of the concentration or chemical gradient across the membrane. It is this gradient that drives the transport of the (relevant) substance with the help of other transporters (e.g. SGLT – Sodium Glucose Transporter).
A transporter carrying out the secondary active transport (SGLT) thus displaces at least two particles – firstly the one, that is supposed to be transported (glucose) and secondly the one, that drives (through the existence of its gradient across the membrane) the transport (Na+). To maintain this gradient, a second transporter is necessary (e.g. Na+/K+-ATPase), which can be located in other portion of the membrane. It is this second transporter that consumes the energy (ATP) – that is why this kind of transport mechanism belongs to the group of active transport. In parentheses, we’ve provided an example of secondary active transport of glucose through SGLT transporter, which uses the gradient of Na+ created by Na+/K+-ATPase. According to the direction of the transport we recognise symport (both particles are transported in the same direction, from or into the cell) and antiport (particles are transported in the opposite direction – one is carried into the cell and the other out of it). SGLT provides the symport of glucose and Na+.
Tertiary active transport works on the similar principle.
3) Transport of macromolecules across the membrane
According to the direction:
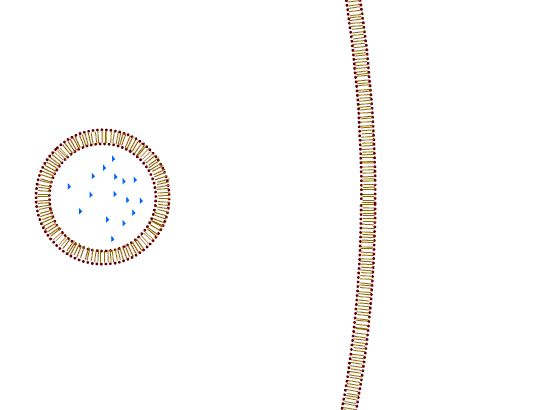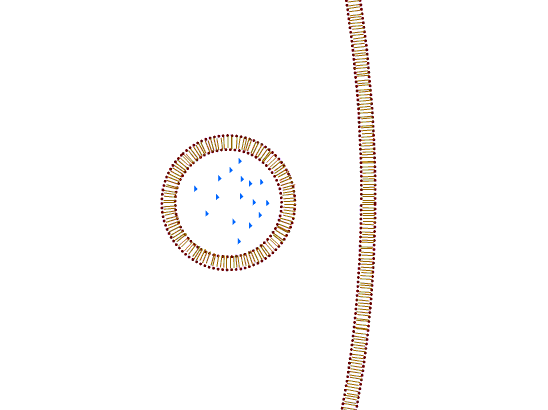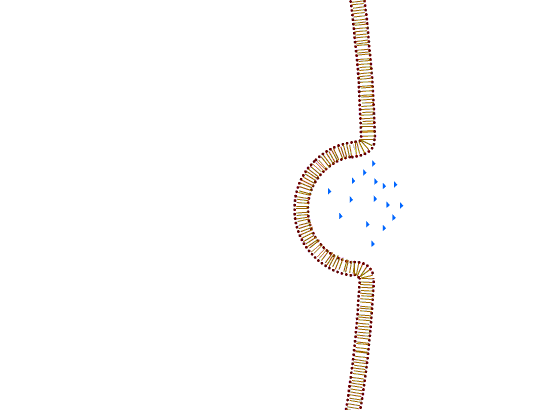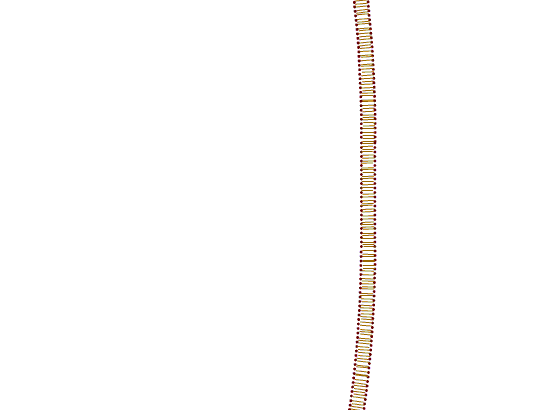
a) Exocytosis is a process by which macromolecules leave the cell. Cytoplasmic membrane fuses with the membrane of the transport vesicle and the macromolecule is either released in the extracellular space or remains a part of the cell membrane.
b) Endocytosis is the process of uptake of macromolecules into the cells. Cytoplasmic membrane invaginates inwards and creates a transport vesicle. According to the nature of the transported particles, the process is carried out differently:
1. Pinocytosis: the transport of macromolecules in solution. The process can be selective (only occurring through specific membrane receptors) or non-selective (the place of invagination is random).
2. Phagocytosis: ingestion of large particles. The cell initially surrounds the particle with protrusions of the cytoplasmic membrane (pseudopodia) and then encloses it to the vesicle.
_
Intracellular transport
Transport inside the cell usually occurs through:
1) Diffusion: particles dissolved in aqueous medium of cytosol
2) Transport in secretory vesicles: proteins are most commonly formed at rough endoplasmic reticulum, followed by their transport to Golgi apparatus. Secretory vesicles or lysosomes break free from GA and are further transported within the cell. This kind of transport is carried out by motor proteins (dyneins and kinesins) that use the ATP in order to move across the surface of microtubules (dynein moves towards their – end and kinesin towards their + end) carrying the vesicle attached to their second end.
_
Compartmentalization of metabolic pathways
Cytosol (cytoplasm without organelles):
1) Metabolism of saccharides: glycolysis, part of gluconeogenesis, glycogenolysis and synthesis of glycogen, pentose cycle
2) Metabolism of fatty acids: FA synthesis
3) Metabolism of amino acids: synthesis of nonessential AA, some of the transamination reactions
4) Other pathways: parts of heme and urea synthesis pathways, metabolism of purines and pyrimidines
Mitochondria:
1) Metabolism of saccharides: PDH, part of gluconeogenesis (conversion of pyruvate to OAA)
2) Metabolism of fatty acids: beta-oxidation of FA (Linen’s spiral), synthesis (hepatocytes only) and degradation (extrahepatic tissues) of ketone bodies
3) Metabolism of amino acids: oxidative deamination, some of the transamination reactions
4) Other pathways: Krebs cycle, respiratory chain and oxidative phosphorylation, parts of heme and urea synthesis pathways
gER:
1) Proteosynthesis (translation of mRNA)
2) Posttranslational modifications (oxidations, cleavage, methylations, phosphorylations, glycosylations)
hER:
1) TAG and phospholipid synthesis
2) FA elongation (to a maximal length of 24 carbon atoms – in nerve tissue) and desaturation (maximally at 9th carbon atom – counted from carboxyl group)
3) Parts of steroid synthesis pathway
4) Biotransformation of xenobiotics
5) Conversion of glucose-6-phosphate to glucose (only in tissues with glucose-6-phosphatase)
GA:
1) Posttranslational modifications (glycosylations, …)
2) Proteins sorting and formation of secretory vesicles
Lysosomes:
1) Hydrolysis of proteins, saccharides, lipids and nucleic acids
Peroxisomes:
1) Degradation of long-chain FA (> 20 carbon atoms)
_
Subchapter Author: Petra Lavríková