Content:
1. Introduction to degradation of lipids and ketone bodies metabolism
2. Lipids as source of energy – degradation of TAG in cells, β-oxidation of fatty acids
3. Synthesis and utilisation of ketone bodies
_
Introduction to degradation of lipids and ketone bodies metabolism
Triacylglycerol (TAG) contain huge amounts of chemical energy. It is very profitable to store energy in TAG because 1 g of water-free TAG stores 5 times more energy than 1 g of hydrated glycogen. Complete oxidation of 1 g of TAG yields 38 kJ, 1g of saccharides or proteins only 17 kJ. Man that weighs 70 kg has 400 000 kJ in his TAG (that weight approximately 10,5 kg). This reserve of energy makes us able to survive starving in weeks. TAG accumulate predominantly in adipocyte cytoplasm.
There are more types of fatty acid oxidation. Individual types can be distinguished by different Greek letters. Greek letter denote atom in the fatty acid chain where reactions take place. β-oxidation is of major importance, it is localised in mitochondrial matrix. ω- and α- oxidation are localised in endoplasmic reticulum.
Animal cells cannot convert fatty acids to glucose. Gluconeogenesis requires besides other things (1) energy, (2) carbon residues. Fatty acids are rich source of energy but they are not source of carbon residues (there is however one important exception, i.e. odd-numbered fatty acids). This is because cells are not able to convert AcCoA to neither pyruvate, nor OAA. Both carbons are split away as CO2. PDH is irreversible. Plant cells are capable of conversion of AcCoA to OAA in glyoxylate cycle.
_
Lipids as source of energy – degradation of TAG in cells, β-oxidation of fatty acids
Lipids are used for energy production, this process take place in 3 phases:
1) Lipid mobilisation – hydrolysis of TAG to FA and glycerol. FA and glycerol are transported in blood.
2) FA is activated in cytosol and then transported to the mitochondrial matrix.
3) β-oxidation: FA is decomposed to AcCoA. AcCoA then enters (1) the TCA cycle, or (2) ketone bodies synthesis.
Lipid mobilisation – lipolysis
Storage lipids are mobilised by the enzyme hormone sensitive lipase (HSL). HSL catalyses this reaction:
TAG → 3 FA + glycerol
Free fatty acids bind to serum albumin, which transports them in blood (e.g. to the liver). Glycerol is transported in plasma, freely soluble.
Lipolysis regulation
HSL is under strict hormone control. Activity of HSL is increased by phosphorylation. Insulin is anabolic hormone, therefore it is clear that insulin inhibits HSL. Glucagon, catecholamines, thyroidal hormones activate HSL.
Use of glycerol
Hydrolysis of TAG provides glycerol. Glycerol can be converted to intermediates of glycolysis/gluconeogenesis. First step is phosphorylation producing glycerol-3-P catalysed by glycerol kinase. Then dehydrogenation take place yielding dihydroxyacetone phosphate, this step is catalysed by the enzyme glyceraldehyde-3-phosphate dehydrogenase. You should know that DHAP is an intermediate of glycolysis/gluconeogenesis.
Entry of fatty acid to the cells
The length of the chain of the fatty acid is crucial factor for manner of entering to the cells. Short chain fatty acids (i.e. less than 12C) can enter by simple diffusion. Fatty acids with longer chain use transport systems – facilitate diffusion (e.g. FATP (fatty acid transport protein) or FAT/CD36 (fatty acid translocase)).
Fatty acids activation
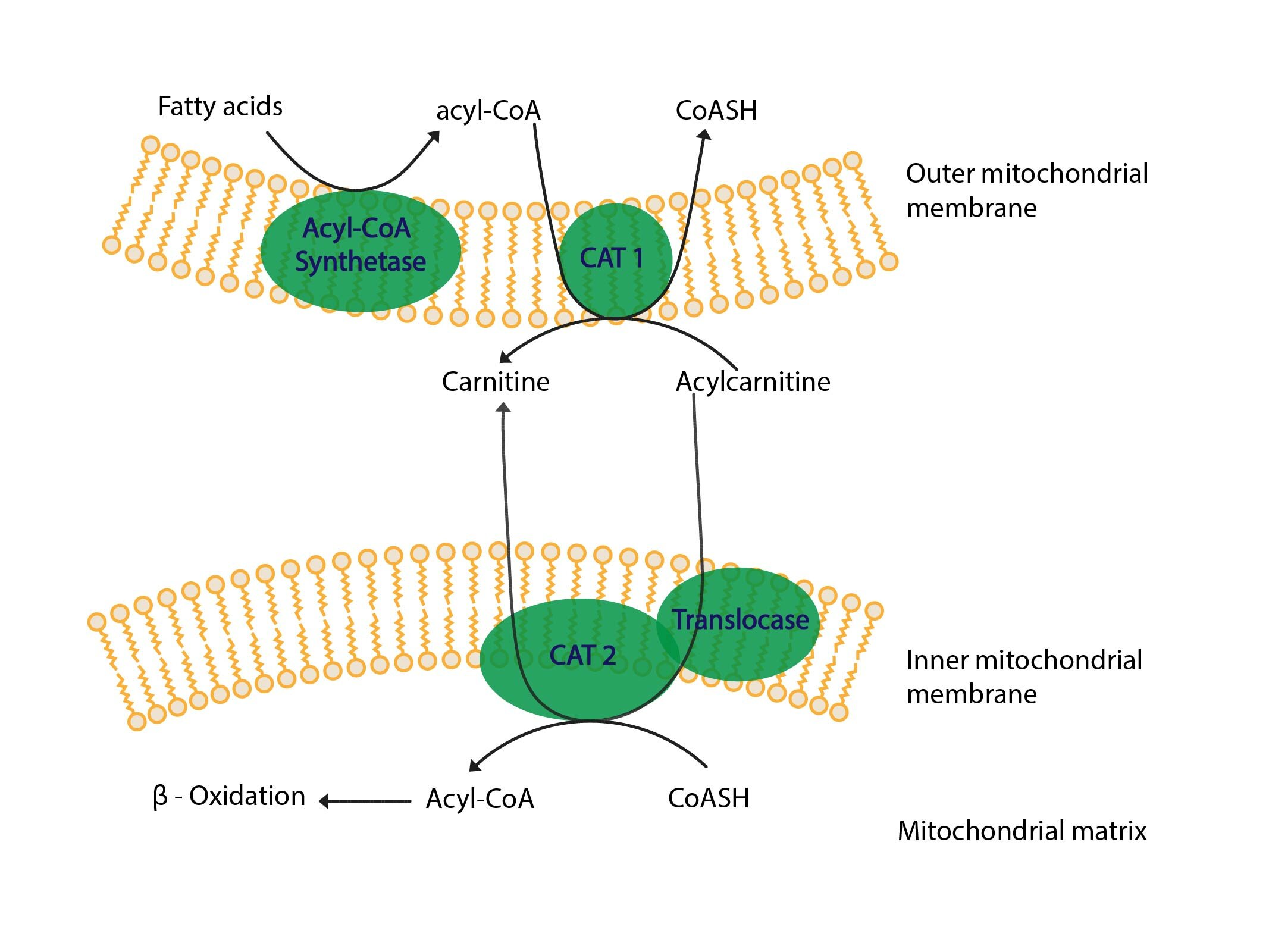
Fatty acids are activated immediately after entering cells. This takes place in cytosol on outer mitochondrial membrane. Non-activated fatty acids cannot enter any metabolic pathway. Activation keeps steady concentration gradient (analogy is glucose phosphorylation – see: Subchapter 2/9) Acyl-CoA synthetase (thiokinase of fatty acids) makes an ester bond between fatty acid and SH-group of co-enzyme A:
FA + ATP + HS-CoA → acyl-CoA + AMP + 2 Pi
Fatty acid activation in fact has two phases. At first acyl-adenylate (acyl-AMP) is produced, secondly AMP is changed for coenzyme A.
Entry of fatty acid to the mitochondrial matrix
Manner of entry of fatty acids to mitochondrial matrix depends (again) on length of their chain:
1) Shorter than C10 enter matrix freely
2) C12-C18 use carnitine transporter
3) Longer than C18 cannot enter mitochondrial matrix
Acyl-CoA with C12-C18 are able to pass outer mitochondrial membrane, inner membrane is however impermeable therefore fatty acid must split up with CoA and bind to carnitine. This reaction, i.e. transfer of fatty acid from acyl-CoA to acyl-carnitine, is catalysed by the enzyme carnitine acyltransferase I (CAT I = carnitine palmitoyltransferase I = CPT I). This enzyme is localised on cytosolic side of outer mitochondrial membrane.
Carnitine-acylcarnitine translocase exchanges carnitine for acylcarnitine in inner mitochondrial membrane. Hence acylcarnitine gets into the mitochondrial matrix.
Carnitine acyltransferase II (CAT II) transfers fatty acid from acylcarnitine to coenzyme A in mitochondrial matrix. This yields carnitine and acyl-CoA. Carnitine is substrate for carnitine-acylcarnitine translocase. Acyl-CoA is a substrate for β-oxidation.
β-oxidation of fatty acids
β-oxidation is closely connected with the ETC, thus it is obvious that β-oxidation take place only in aerobic conditions. Single steps of the β-oxidation are catalysed by 4 enzymes:
1) Acyl-CoA dehydrogenase – prosthetic group is FAD.
2) Enoyl-CoA hydratase
3) L-3-hydroxyacyl-CoA-dehydratase – NAD+ is co-enzyme
4) β-ketothiolase
β-oxidation can be summarised as follows: dehydrogenation-hydration-dehydrogenation-thiolytic cleavage. First three steps are the same as in the TCA cycle as follows: (see Subchapter 2/8)
1) Oxidation of succinate to fumarate catalysed by the enzyme succinate dehydrogenase – cofactor is FAD.
2) Adding water to double bond in fumarate, thus yielding malate; catalysed by the enzyme fumarate hydratase.
3) Malate oxidised to oxaloacetate, catalysed by the enzyme malate dehydrogenase – cofactor is NAD+.
Acyl-CoA-dehydrogenase – first oxidation
This enzyme catalyses generation of double bond between second (α) and third (β) carbon in fatty acid chain. This reaction is stereospecific, thus trans-enoyl-CoA is produced. FAD accepts electrons. Different chain lengths need different dehydrogenases. There are 3 main groups of dehydrogenases according to chain length:
1) Short chain fatty acid (4-6C)
2) Medium chain fatty acid (6-10C)
3) Long chain fatty acid (12-18C)
Enoyl-CoA-hydratase
This enzyme catalyses hydration of trans-double bond made in first step. Hydroxyl group is produced in L-3-hydroxyacyl-CoA.
Hydroxyacyl-CoA-dehydrogenase
This enzyme catalyses oxidation of hydroxyl group on third (β) carbon and keto group is thus produced. NAD+ accepts electrons.
β-ketothiolase
This enzyme catalyses thiolytic splitting. This is the last step of β-oxidation turnover. In this reaction β-keto carbon is attacked by SH-group of co-enzyme A. Co-enzyme A is thus attached to β-keto carbon and (1) 2-carbons shorter acyl-CoA and (2) AcCoA are produced.
One turnover of the β-oxidation
β-oxidation is cyclic process. One turnover could be described like this:
Acyl-CoA + FAD + NAD+ + HS-CoA → acyl-CoA (2-carbons shorter) + FADH2 + NADH + H+ + AcCoA
An intermediate (acyl-CoA 2-carbons shorter) enters next turnover of the β-oxidation. Majority of fatty acids have even numbered carbon chain, thus in the last turnover butyryl-CoA is converted to two molecules of AcCoA.
Yield of complete palmitate oxidation
Overall yield and energetic balance of complete palmitate oxidation in equation follows:
Palmitoyl~CoA + 7 FAD + 7 NAD+ + 7 HSCoA + 7 H2O → 8 AcCoA + 7 FADH2 + 7 NADH+H+
As admitted in Subchapter 2/7 we are not able to determine precise amounts of ATP produced in the ETC. Following values are only approximate and we show them just make you able to compare energy balance of glucose oxidation with fatty acid oxidation. In the ETC one NADH provides 2,5 (3) ATP and one FADH2 provides 1,5 (2) ATP. In summary:
1) 7 x FADH2 = 10, 5 (14) ATP
2) 7 x NADH = 17, 5 (21) ATP
3) 8 AcCoA oxidation in the TCA cycle provides 80 (96) ATP
Overall yield is 108 (131) ATP, for fatty acid activation 2 ATP were utilised, thus net profit is 106 (129) ATP.
Regulation of β-oxidation of fatty acids
β-oxidation is regulated on the level of entry of fatty acids to mitochondrial matrix, i.e. on the level of carnitine transporter, more precisely on the level of carnitine acyltransferase I. Carnitine acyltransferase I is inhibited by intermediate of fatty acid synthesis, i.e. malonyl-CoA. This is so called cross-regulation. Mechanism of cross-regulation is as follows: fatty acid synthesis is localised in cytosol, carnitine acyltransferase I reaction as well. Malonyl-CoA is product of the first reaction of fatty acid synthesis. Cross-regulation thus prevents synthesis and degradation to run at the same time.
Insulin inhibits β-oxidation, counterregulatory hormones activate it.
Odd-numbered fatty acids
Oxidation of odd-numbered fatty acid produce both AcCoA, and propionyl-CoA. Propionyl-CoA is carboxylated to methylmalonyl-CoA that is converted to succinyl-CoA (succinyl-CoA is the TCA cycle intermediate). Succinyl-CoA is converted in the TCA cycle to oxaloacetate, and oxaloacetate could be substrate for gluconeogenesis – i.e. odd-numbered fatty acids can be converted to glucose. This type of fatty acids is however quite rare in the body.
Degradation of unsaturated fatty acids
Majority of unsaturated fatty acids contain cis-configuration of double bonds. These fatty acids are degraded in the β-oxidation. Only difference is that enoyl-CoA-hydratase requires trans-isomers, thus cis-isomers must be converted to trans–isomers by the enzyme isomerase.
Oxidation of very long chain fatty acids
Very long chain fatty acids (longer than 18C) oxidation takes place in peroxisomes. First step is catalysed by flavoprotein dehydrogenase that transfers electrons to O2, thus H2O2 is produced:
1) FADH2 from first step is not reoxidised in the ETC but in reaction with O2:
FADH2 + O2 → FAD + H2O2
2) Peroxisomal catalase cleaves H2O2:
2 H2O2 → 2 H2O + O2
Oxidation terminates when octanoyl-CoA is produced. Octanoyl-CoA is transported bound to carnitine to β-oxidation. Reactions described above do not lead to ATP production.
α-oxidation a ω-oxidation
These pathways are of minor importance. In ω-oxidation reactions take place on the last carbon. In α-oxidation oxidation takes place on α-carbon.
_
Production and utilization of ketone bodies
Production and function of ketone bodies
Ketone bodies are acetoacetate, β-hydroxybutyrate and acetone. Ketone bodies are produced predominantly in hepatocytes mitochondria. Ketone bodies are water soluble transport form of acetyls. Ketone bodies are produced when excessive acetyl-CoA is present. Acetyl-CoA is produced in hepatic β-oxidation, i.e. the liver pre-arrange fatty acids and provide ketone bodies as an alternative source of energy.
Enter of AcCoA to the TCA cycle depends on availability of oxaloacetate. Oxaloacetate is produced by pyruvate carboxylation. In starving or in diabetes mellitus OAA is utilised in gluconeogenesis. Lack of saccharides leads to decreased OAA and thus the TCA is decelerated.
Before ketogenesis (i.e. ketone bodies synthesis) is described, situation when ketogenesis is activated will be depicted. At first lipogenesis is activated by hormone sensitive lipase (HSL). Lipogenesis leads to increased concentration of plasmatic fatty acid. Therefore fatty acids enter hepatocytes. In hepatocytes β-oxidation takes place and excess of AcCoA is produced. Hepatocyte is not able to use AcCoA in other pathways and that is reason why AcCoA enters ketogenesis. Source of carbon atoms for ketogenesis is only AcCoA.
Course of ketone bodies synthesis is as follows:
1) Two molecules of AcCoA condensation → acetoacetyl-CoA
2) Acetoacetyl-CoA reacts with next AcCoA → 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA)
3) HMG-CoA cleavage → AcCoA + acetoacetate
4) Reversible interconversion of acetoacetate and β-hydroxybutyrate
5) Acetoacetate decarboxylation
β-Ketothiolase
β-ketothiolase catalyses the last step of β-oxidation of fatty acids – thiolytic cleavage. In ketone bodies synthesis the reaction takes place in reverse course and thus two molecules of AcCoA is converted to acetoacetyl-CoA. This step is localised in mitochondrial matrix.
3-hydroxy-3-methylglutaryl-CoA synthase
This enzyme catalyses condensation of acetyl-CoA with acetoacetyl-CoA. Condensation is localised on the third carbon of acetoacetyl-CoA and thus 3–hydroxy-3-methylglutaryl-CoA is produced. HMG-CoA is very important intermediate also in cholesterol synthesis.
3-hydroxy-3-methylglutaryl-CoA lyase
This enzyme catalyses cleavage of HMG-CoA to acetoacetate and AcCoA. In this step first ketone body (i.e. acetoacetate (AcAc)) is produced
β-hydroxybutyrate dehydrogenase
This enzyme catalyses mutual reversible conversion of two ketone bodies – acetoacetate and β-hydroxybutyrate. Cofactor is NAD+. In conditions when massive production of ketone bodies takes place β-hydroxybutyrate is quantitatively the most important ketone body in the blood; i.e. majority of acetoacetate is converted to β-hydroxybutyrate.
Acetoacetate decarboxylation
Some of acetoacetate molecules are spontaneously (non-enzymatically) decarboxylated to acetone. The body is unable to use acetone at all, thus it is eliminated by breathing and in the urine.
Activation and utilization of ketone bodies
Ketone bodies are polar, i.e. they are transported freely in plasma. Ketone bodies are utilized only outside the liver, since hepatocytes do not contain enzyme required for ketone bodies activation. At first oxidation of β-hydroxybutyrate to acetoacetate. Acetoacetate is activated by transfer of coenzyme A from succinyl-CoA, thus acetoacetyl-CoA is produced. Acetoacetyl-CoA is converted to AcCoA (this is part of β-oxidation, catalysed by the enzyme thiolase). AcCoA enters the TCA cycle.
The heart muscle, skeletal muscle and the kidney cortex prefer ketone bodies oxidation rather than glucose oxidation. The brain in starving adapts to ketone bodies oxidation – in long-term oxidation starving brain covers 50% of its energy demand by ketone bodies oxidation.
Ketogenesis regulation
Ketogenesis regulation is performed in four levels:
1) Hormone sensitive lipase – lipolysis in the adipose tissue
2) Carnitine acyltransferase I – entry of fatty acids to the mitochondrial matrix, where β-oxidation is performed
3) AcCoA from β-oxidation to ketogenesis rather than to the TCA cycle
4) Mitochondrial HMG-CoA-synthase
High ketone bodies level in the blood indicates presence of great amounts of AcCoA. Its consequence is lipolysis inhibition.
Ketone bodies plasma concentration and ketoacidosis
Summary of plasma concentration of 3-hydroxybutyrate (3-HB) in different situations follows:
1) After a meal: 3-HB ~ 0,05 mmol/l, FFA < 0,2 mmol/l
2) 12 hours fasting: 3-HB < 0,2 mmol/l, FFA ~ 0,4 mmol/l
3) 21 days starving: 3-HB ~ 5 mmol/l, FFA ~ 1,5 mmol/l
4) T1DM, developed ketoacidosis with pH = 7,0: 3-HB ~ 20 mmol/l, FFA ~ 5 mmol/l
Maximal velocity of ketone bodies production is achieved when plasma concentration is ~ 12 mmol/l.
Ketoacidosis
Ketoacidosis is a condition when plasma concentration of ketone bodies increase. Since ketone bodies are quite strong acids their increased concentration lead to the acidemia (decreased pH). This condition is potentially life-threatening. Diabetic ketoacidosis (DKA) is caused by insulin deficiency. Low ketoacidosis causes decreased pH in starving. Next condition associated with ketoacidosis are as follows:
1) Alcoholic ketoacidosis
2) Gravidity ketoacidosis
3) Poisoning: isopropyl alcohol, salicylates
4) Congenital metabolic disorders
Diabetic ketoacidosis (DKA)
Relative excess in counterregulatory hormones and deficiency in insulin are pathophysiologic base of DKA. This leads to excessive lipolysis in the adipose tissue, that leads to increase in plasma fatty acid concentration. In hepatocytes malonyl-CoA concentration decreases, this stops inhibition of transport of acyl-CoA to mitochondrial matrix and thus β-oxidation is launched. HMG-CoA synthase is activated and the ETC is saturated by reduced cofactors from β-oxidation, hence the TCA cycle is decelerated. Simultaneously production and utilization of ketone bodies increases. Ketone bodies are present in the urine – ketonuria. Utilization of ketone bodies is maximal when concentration is approximately 12 mmol/l. Their accumulation takes place and acidemia deepens. In the breath is sweetish odour of acetone.
_
Subchapter Authors: Josef Fontana and Petra Lavríková