Content:
1. Overview of saccharides
2. Overview of lipids
3. Overview of proteins
_
Overview of saccharides
Classification and structure
Saccharides (from Latin saccharum = sugar, also called carbohydrates) are the most abundant substances on the Earth. Their molecules are composed of atoms of hydrogen, carbon and oxygen. In chemical terms, they are polyhydroxy aldehydes and polyhydroxy ketones, which mean that their molecules contain, apart from aldehydic and ketonic functional groups, multiple hydroxyl functional groups.
Classification of saccharides
According to the number of units we can distinguish: monosaccharides (that can not be further hydrolysed to simpler units), oligosaccharides (that, when hydrolysed, produce 2-10 monosaccharide units) and polysaccharides (that can be hydrolysed to more than 10 monosaccharides). Monosaccharides and oligosaccharides are referred to as “sugars”. The synonym for polysaccharide is glycan.
Monosaccharides can be further divided according to:
1) The number of C-atoms: trioses, tetroses, pentoses, hexoses, etc
2) The functional group: aldoses and ketoses
Polysaccharides are divided into:
1) Homopolysaccharides: are polymers consisting of the same kind of monosaccharide. Examples are starch, glycogen or cellulose.
2) Heteropolysaccharides: are polymers consisting of more than one kind of monosaccharide. Example is hemicellulose.
The molecular structure can be expressed in variety of projections:
1) Linear (Fischer)
2) Cyclic (Haworth): that is produced by creating a heterocyclic structure. The ring can be six-membered – called pyranose (for its resemblance to six-membered ring structure of a pyran) or five-membered – called furanose (resembling five-membered structure of a furan).
3) Tollens projection depicts the transformation of a linear projection into the cyclic one, involving a reaction between hydroxyl and carbonyl group, forming a hemiacetal structure.
An isomerism is a state, when molecules with the same molecular formula have different chemical structures. The most common types of isomerism in saccharides include:
1) D- and L-series: the assignment of D or L is made according to the position of the OH- group on the last asymmetric (chiral) carbon atom from the carbonyl group. The assignment is based on the similarity of the molecule with the molecule structure of the first compound of the saccharide series – glyceraldehyde (in Fischer projection, the OH- group is on the right for the D-series and on the left for the L-series). The monosaccharides commonly occurring in our bodies belong to D-series and the enzymes catalysing their transformation are stereospecific to these isomers.
D- and L-isomers are mirror images (so-called enantiomers or optical isomers) and they can differ in the direction of the rotation of plane-polarized light. We cannot generally say that D-series rotates the plane-polarized light to the right and L-series to the left. Each of them can be + (right-rotatory) or – (left-rotatory). An equimolar mixture of enantiomers (or D- and L-isomers) is termed racemic mixture (or DL mixture) and has zero net rotation of the plane-polarized light.
2) Pyranoses and furanoses are termed according to the resemblance of the cyclic form of particular monosaccharide to the ring of the pyran or furan. More than 99 % of glucose molecules in solution exist in the form of gluco-pyranose, but there is still a portion of glucose (though less than 1 %) that has the form of glucofuranose.
3) α- and β-anomers are terms given according to the position of the hemiacetal or hemiketal OH- group within the ring. If this OH- group is located on the same side as the OH- group indicating the D- or L-series, we call the anomer a and if it is on the opposite side, we call the anomer b. D-series a-anomers have the OH- group positioned below the plane of the ring.
Anomers differ in their ability to rotate plane-polarized light. For example, when dissolving the crystalline sugar in water, equilibrium between two anomers is reached (and is accompanied with a change in the rotation of the plane-polarized light). This phenomenon is called mutarotation.
4) Epimeres are isomers that differ from each other in a position of only one OH- group within the molecule.
Examples are galactose (4-epimere of glucose) and mannose (2-epimere of glucose).
5) Aldoses and ketoses differ in the type of the functional group on 1. and 2. carbon atom of the molecule.
The importance for human body
Saccharides are not essential for humans and are commonly synthesized from various molecules like amino acids or glycerol.
The main role of monosaccharide and disaccharides is in being the main source of energy for cells (as an energy source, they are inevitable for erythrocytes and neural cell). Polysaccharides (like glycogen in animals) function as an energy reservoir. Saccharides have important structural function as well – they are part of glycoproteins and glycolipids located in membranes, they are inevitable for nucleic acids and coenzyme synthesis and they form an important part of extracellular matter (for example by being a part of proteoglycans).
Overview
1) Monosaccharides and disaccharides
In general they are white, crystalline substances soluble in water, neutral in nature (they do not dissociate in water solutions). They have polar character and the OH- groups are responsible for their sweet taste and strong hydration in solutions.
The most important saccharides found in diet are glucose, fructose and galactose. Concerning disaccharides, the most important ones are sucrose (α-Glc (1→2) β-Fru) used as a sweetener (table sugar), lactose (β-Gal (1→4) β-Glc) found in milk and maltose (α-Glc (1→4) β-Glc) found in malt.
The most important derivatives of monosaccharides include:
a) Sugar alcohols
Sugar alcohols are formed through the reduction of carbonyl functional group into the hydroxyl group. For example glucitol (also called sorbitol) is formed by reduction of glucose or fructose.
Clinical correlation:
A diabetic cataract is a result of a long-term elevation in the glucose concentration. Inside the lens glucose reduces to osmotically active glucitol that changes the osmolarity within the lens. As it is only slowly removed, the proteins of the lens (called crystallines) denature and form deposits that strongly diffuse the light.
b) Polyhydroxy derivatives of carboxylic acids
They are created by oxidation of monosaccharides. When reacting with a weak oxidising agent, only the aldehydic group is oxidised and aldonic acids are produced. Stronger oxidising agents oxidise not only the aldehydic, but the primary hydroxy group at the end of the molecule as well. The resulting dicarboxylic acids are called aldaric acids. The oxidation of only the primary hydroxyl group of aldoses is possible as well. The reaction is in human body catalysed by enzymes and uronic acids are formed. For example glucose is oxidised to form a glucuronic acid, an important conjugating agent, that helps to excrete in water poorly soluble substances.
c) Deoxy sugars
Deoxy sugars are created by a reduction of the hydroxyl group of the saccharides. The most important deoxy sugar is deoxyribose, an important part of nucleic acids.
d) Amino sugars
Are formed by the substitution of hydroxy group for NH2-group. Important amino sugar is D-glucosamine, constituent of the molecules found in extracellular matter.
e) Esters
Esters are formed by esterification, a reaction between hydroxyl group and H3PO4 (for example glucose-6-phosphate, derivative of glucose) or H2SO4 (part of the proteoglycans).
f) Glycosides
They can be formed by reaction between the OH- group and:
1. Alcohol (O-glycosidic bond): an example is a creation of disaccharides or polysaccharides or a bond between monosaccharides and proteins through Ser and Thr amino acids.
2. Amine (N-glycosidic bond): an example is a bond between the monosaccharide and protein through Asp or binding of ribose in nucleotides.
Substances that bind to the monosaccharides by the mean of the glycosidic bond, but themselves are not saccharides are called aglycones.
The most reactive group in the molecule of monosaccharide is the anomeric hydroxyl group. When a glycosidic bond between anomeric OH- groups of two monosaccharides is created, the resulting disaccharide is non-reducing (it does not react with oxidising agent). In the case of a reaction between the anomeric hydroxyl group of one monosaccharide and other hydroxyl group of the second one, the disaccharide produced is reducing. Free aldoses (monosaccharides) are all reducing, examples of reducing disaccharides are lactose and maltose.
2) Polysaccharides and fiber
Polysaccharides are amorphous substances, either not soluble in water or creating colloid mixtures. They are termed glycanes and they can be made of one kind of monosaccharide only (for example glucose in the case of starch and glycogen) and we call the glucans, fructans, etc or are made of different kinds of monosaccharides and their derivatives (for example glycosaminoglycanes).
The storage polysaccharides, like starch or glycogen, are partially soluble in water, whereas the structural polysaccharides, like cellulose, that have lots of intra- or intermolecular hydrogen bonds, are insoluble in water.
The fiber is a term for a relatively heterogeneous group of structural polysaccharides that the human enzymes are not able to cleave. Therefore it forms a non-absorbable, but nevertheless an important part of our diet. It increases the chyme volume and thus speeding up the intestinal peristalsis (decreasing the time toxic substances are in contact with the intestinal epithelium). Fiber also binds different exogenous and endogenous substances and facilitates their elimination. Important example are bile acids, that are formed from cholesterol and so their elimination aids its reduction in the body. To learn more about fiber check Subchapter 9/3.
The fiber can be divided into:
a) Soluble fiber (hemicellulose, pectines) is a portion of a fiber that can be cleaved by bacteria in a large intestine. The resulting short-chain fatty acids (acetic, propionic, butyric acid) are important energy source for colonocytes.
b) Non-soluble (cellulose): that even bacteria cannot cleave and so leaves the body undigested. Its importance lies in increasing the volume of the chyme and in supporting the peristaltic movements.
3) Heteroglycosides
Apart from molecules made purely of saccharides, there exist substances consisting of saccharides and other compounds (aglycones). These are called heteroglycosides and examples include:
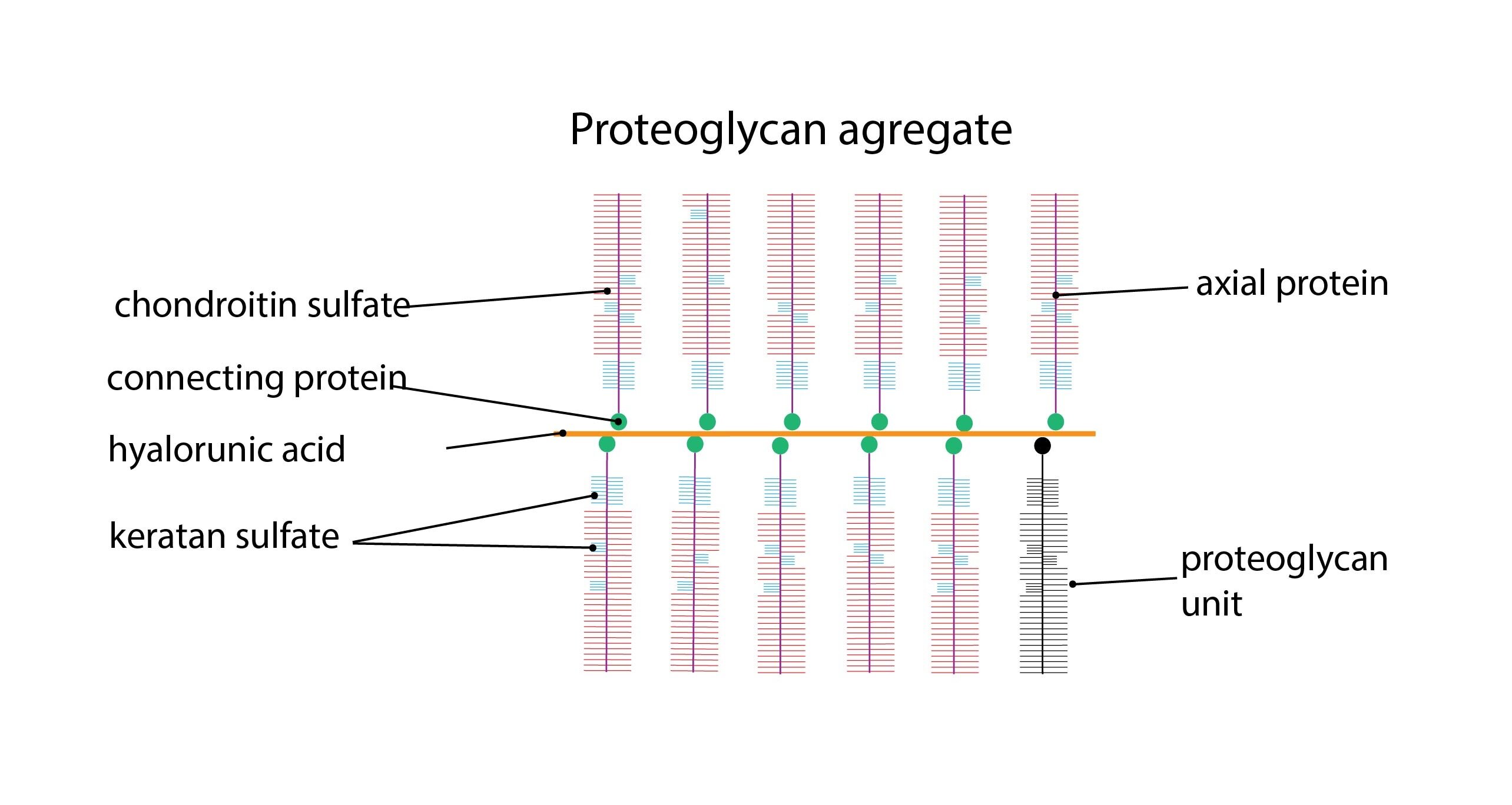
a) Proteoglycans contain long linear polysaccharide chains (that make up most of the molecule) bind to the protein. The chains consist of repeating amino-sugar and uronic acid dimers that are called glycosaminoglycanes.
b) Glycoproteins are proteins that have various parts of the molecule glycosylated (through O- or N- glycosidic bond) by short-branched molecules of oligosaccharides. Unlike proteoglycans, they do not contain uronic acids.
c) Glycolipids are substances of lipid nature, that have one or several monosaccharide units attached to their molecule.
_
Overview of lipids
Classification and structure
Lipids are a group of chemically and structurally heterogeneous substances that share some characteristics. They are hydrophobic (and so poorly soluble in water and very well soluble in nonpolar solvents), their molecules contain alcohols and fatty acids and their biosynthesis usually starts with acetyl-CoA.
By term fatty acids we usually understand higher monocarboxylic acids (having 8 and more C atoms), that typically have odd number of carbon atoms. If they contain double bonds, they are usually isolated and in cis-configuration. Most of the fatty acids have 16 and 18 C atoms.
Classification of lipids
1) Simple lipids: are lipids being made of hydrophobic parts only, they are esters of fatty acids (FA) and various alcohols.
a) Acylglycerols (glycerides): are esters of higher FA and glycerol.
According to the number of FA bound to the alcohol we recognise mono-, di- or triacylglycerols. The most important for humans are triacylglycerols that are part of fats (which are mixtures of solid triacylglycerols) and oils (mixtures of liquid triacylglycerols).
Acylglycerols undergo acidic hydrolysis and turn back to corresponding fatty acids and glycerol. Products of alkalic hydrolysis (so-called saponification) are glycerol and a mixture of salts of the fatty acids (soaps).
b) Waxes: are esters of higher FA and higher monohydric alcohol (for example cetyl alcohol with 11 carbons, ceryl alcohol with 22 C or myricyl alcohol with 32C). Waxes are very resistant towards hydrolysis and therefore cannot be digested by humans.
2) Complex lipids: apart from hydrophobic part, they contain hydrophilic components as well, which results in their ability to form micelles and double layers (and so are the fundamental constituent parts of cell membranes). They are also called polar lipids.
a) Phospholipids: contain part of the phosphoric acid molecule (H3PO4).
1. Phosphoacylglycerols: are made of glycerol molecule esterified with two FA and one molecule of phosphoric acid, giving rise to phosphatidic acid. Other substituents can bind to its phosphate group (choline, serine, …). The most abundant group of phosphoacylglycerols are phosphatidylcholines (lecithines), that are found in biological membranes.
2. Sphingomyelins: contain specific alcohol called sphingosine, with other constituents. Sphingosine with bound FA is termed ceramide. Sphingomyelins occur for example in nerve tissue.
b) Glycolipids: contain one or more monosaccharide units that is bound by a glycosidic bond to a lipid part of the molecule (mono-, diacylglycerol or sphingosine).
1. Cerebrosides: their molecule consist of ceramide with one bound FA and galactose. Cerebrosides are abundant in white matter of the brain.
2. Gangliosides: their molecule consists of ceramide with oligosaccharide (usually made of galactose and glucose). Gangliosides are present in ganglia of nerve cells and in gray matter of the brain.
c) Lipoproteins: are a combination of lipids and proteins.
Essential FA
Human body can desaturate the molecule of fatty acid only up to the 9th carbon atom. If the double bond occurs further in the molecule, the body is not able to synthesise and such FA must be obtained from a diet (and so is essential for human body).
The main essential FA is linoleic acid (18:2, with cis double bonds at 9 and 12 positions – that is why it is termed as ω-6) that can be found mainly in vegetable oils (like sunflower oil). Linoleic acid is used in biosynthesis of arachidonic acid (20:4; ω-6), that is an important precursor molecule of many important biologically active substances (for example prostaglandins, prostacyclins, leukotrienes and thromboxanes). Through their action is linoleic acid responsible for proinflammatory effects and increases the level of certain plasmatic lipids. α-linolenic acid (18:3, with cis double bonds at positions 9, 12 and 15 – termed as ω-3) can be mainly found in sea fish and other marine animals. Unlike the linoleic acid it reduces the plasmatic levels of cholesterol and TAG and so decreases the risk of cardiovascular diseases. It has anti-inflammatory effects as well.
Importance for human body
Lipids are the most reduced and so energetically richest nutrient. That is why they are important energy source for human body. However, there are tissues (like nerve tissue) that are unable to use them extensively in their metabolism. Due to their hydrophobic properties, they do not bind water (that would increase their weight) and so are the most effective energy storage molecules. In men, fat forms around 15 % of total body weight, in women the percentage is higher, around 20-25 %. This means, that an average man’s body has around 10.5 kg of TAG, that is able to produce around 400 000 kJ of energy when oxidised.
Apart from having a function in energy metabolism, lipids have an important structural function as they are a part of all cell membranes. Structural and mechanical function consist in providing a thermal insulation (for example subcutaneous fat or fat around organs) and an electrical insulation as well (for example myelin sheaths of neurons).
Lipids are solvents for many non-polar substances (like fat-soluble vitamins) and are starting compounds for biosynthesis of many important substances (eicosanoids, steroid hormones, bile acids etc.)
Isoprenoids
Apart from lipids, our body contains another group of non-polar substances that are base on the molecule of isoprene and contain two or more such molecules in their structure. Isoprenoids can be divided into:
a) Terpenoids, that have whole number of isoprene units.
b) Steroids, derivatives of triterpenoids (containing six isoprene units). The most important representative of steroids is cholesterol, a constituent of cell membranes and precursor molecule for other substances (bile acids, steroid hormones).
_
Overview of proteins
Classification and structure
Proteins are macromolecular organic substances made of a chain of amino acids (AA) bound together through a peptide bond. Through simple covalent bond it binds amino group of one amino acid and carboxyl group of the other amino acid. A long chain of amino acids is formed through polycondensation, ended at one end with free amino group (N-terminus) and at the other end with free carboxyl group (C-terminus).
Peptides differ from proteins in having a shorter AA chain (less than 100 AAs) and their molecular weight is usually lower than 10 000.
Classification of proteins
Proteins can be divided into:
1) Simple: simple proteins consist only of amino acid chain. We recognise fibrillar and globular proteins.
a) Fibrillar proteins (also called scleroproteins)
Fibrillar proteins are not soluble in water and have mainly structural function. The individual peptide chains are interconnected by crosslinks to form parallel filaments. Examples of fibrous proteins are collagen (component of the connective tissue) or keratin (occurring in hair, skin or nails).
b) Globular proteins (spheroproteins)
Globular proteins are proteins with spherically folded chain, that allows the hydrophobic parts of the molecule to be hidden inside the sphere. These proteins are therefore soluble in water. Example is albumin.
2) Complex proteins
Complex proteins consist of protein part as well as other, non-protein part.
a) Glycoproteins containing glycosidically bound saccharide.
b) Metalloproteins containing metal ions (Fe, Cu), like ferritin or transferrin
c) Chromoproteins containing pigment as a prosthetic group, for example haemoglobin, cytochromes or myoglobin.
d) Nucleoproteins containing nucleic acids
e) Lipoproteins containing lipids
The structure of a protein molecule shows different levels:
1) Primary structure
Primary structure is base on the amino acids sequence forming the protein. The sequence is read from N- to C-terminus. Amino acids are bound together through peptide bonds.
2) Secondary structure
Secondary structure is based on the hydrogen bonds between NH- and C=O groups of the peptide bond. The most common secondary structure are alpha-helix and beta-pleated sheet.
a) Alpha-helix
The protein chain forms right- or left-handed helix with the length of one turn being 3.6 amino acid residues. The side chains protrude outward from the helix.
b) Beta-pleated sheet
Consist of two chains, arranged in antiparallel and stabilised with H-bonds.
Apart from these two secondary structures there exist many more, for example Leu- zipper or zinc – finger.
3) Tertiary structure
Tertiary structure describes the spatial arrangement of the molecule based on the interactions between the side chains (electrostatic interactions, H-bonds, SH- bonds, non-polar interactions, etc.)
4) Quaternary structure
Quaternary structure describes the spatial arrangement of the protein subunits in the case of proteins consisting of more than one chain. The subunits are not linked together through peptide bonds.
Denaturation is a process that leads to the disintegration of all higher structures of a protein molecule apart from its primary structure. The protein loses its functionality; only its energetic value is preserved. Denaturation can be caused by high temperature, changes in pH or presence of heavy ions salts.
Amino acids (AA)
Amino acids are the basic building blocks of proteins and peptides. They contain at least one amino (-NH2) and one carboxyl (-COOH) group.
From the biosynthetic point of view, AA can be divided into:
1) Nonessential, that can be synthesized by human body: Gly, Ala, Pro, Ser, Tyr, Cys, Asp, Asn, Glu, Gln
2) Essential that must be present in our diet:
a) Branched: Val, Leu, Ile
b) Aromatic: Phe, Trp
c) Basic: His a Arg (both are essential only during childhood and in critical states), Lys
d) Containing S: Met
e) Containing OH- group: Thr
Importance for human body
The importance of proteins in human body is enormous. The perform various functions: structural (collagen, elastin, …), motoric (actin, myosin, …), informational (protein hormones), protective (immunoglobulins, complement, antigens, …), transport (albumin), catalytic (enzymes) and other. At the same time they provide the only source of organically bound nitrogen.
_
Subchapter Author: Petra Lavríková