Content:
1. Introduction to the synaptic transmission
2. Structure of the synaptic transmission and processes at the synapse
_
Introduction to the synaptic transmission
The brain tissue was for a long time considered to be a syncytium. Old histologists believed that the cytoplasm of each cell is united and the brain is multicellular organ resembling a mushroom.
This conviction was refuted by Santiago Ramón y Cajal, who silvered sections of brain and found that the brain tissue is constituted by discrete cells – neurons, which are separated to each other by thin highly specialized slits, synapses.
Types of synapses
There are two types of synapses – chemical and electrical.
Chemical synapses
Almost every synapse in a human being are chemical. Their characteristics are: the first neuron is secreting a substance at the end of own axon called neurotransmitter, which is registered by a corresponding receptor on a dendrite, body or even axon of the second neuron, where produce specific changes (depending on the type of the receptor).
One of the most important characteristic of the synapse is one way transmission. The one neuron, which secrets a neurotransmitter is called presynaptic and his axon is ending on the next neuron with appropriate receptors in the plasmatic membrane – on the neuron postsynaptic. Although action potential can arise everywhere on the membrane of neuron and then can spread in both directions, it’s fading on dendrite, because there is no molecular – biologic apparatus answering by exocytosis of vesicles containing neurotransmitter. Switching of the neuronal impulse (action potential) to the next neuron becomes only in one way at the ending of axon. We can say that the neuronal impulse is always spreading from the presynaptic neuron to the postsynaptic neuron.
This principle is very important, because it enables nervous system to conduct signal only to specific areas. So impulses can be very effective and specialized.
Electrical synapses
Those are much more rare in the central nervous system. Their function is based on the connections of the single neuron to another by gap junctions (nexus), which allow straight transfer of ions and changes electrical potential of the next neuron. This type of connection is playing crucial role in the heart, where we can find gap junctions in high density in intercalated discs of cardiac muscle cells. Here they allow the heart muscle to contract synchronically.
This type of synapse enables to conduct the signal in both directions, because it’s only open channel in the essence.
_
Structure of the synapse and processes at the synapse
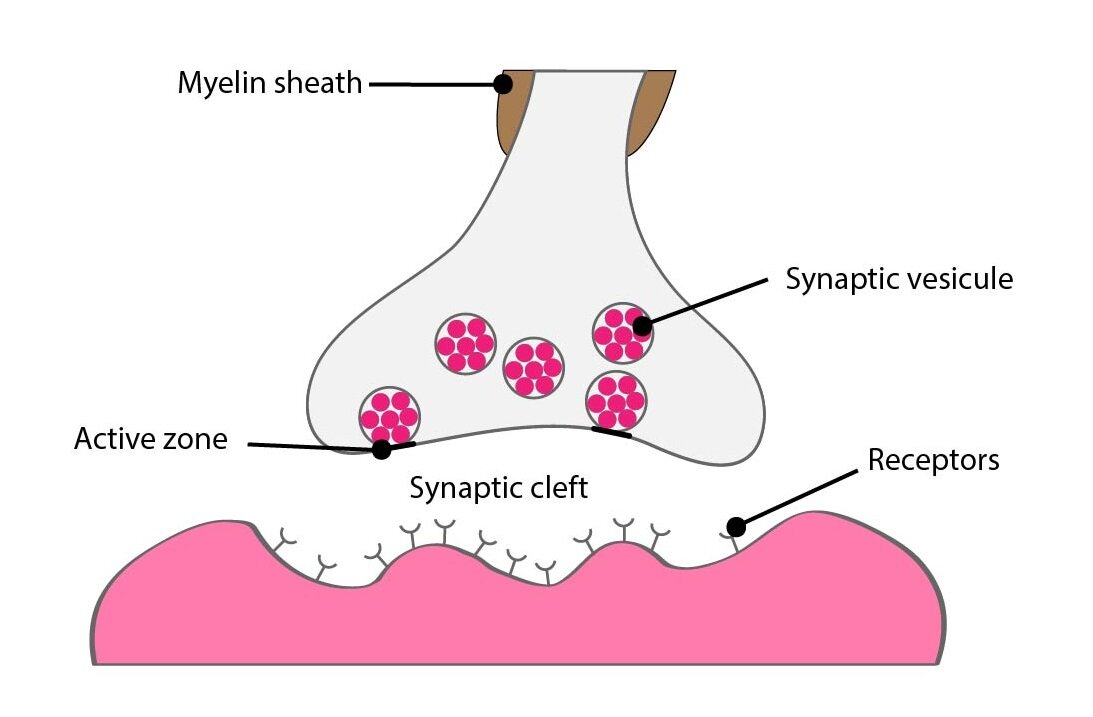
Presynaptic terminal
There are up to 200 000 endings of the presynaptic neuron on body and dendrites of each neuron. 95 % of these endings are on dendrites and we call them presynaptic terminals. They are terminal parts of neuronal axons located in different parts of the central nervous system.
The terminal has a shape of oval nodus and so is sometimes called bouton terminaux. Between presynaptic terminal and dendrite (or body) is 20 nm wide space called synaptic cleft. This cleft isn’t absolutely empty, there is wispy interwoven net of different proteins except the interstitial fluid, many of them have own enzymatic activity.
The presynaptic terminal contains two very important structures: synaptic vesicles in active zone and a large number of mitochondria.
Active zone is modified part of the cell membrane which neighbours with belonging synaptic cleft. It incorporates molecular-biological apparatus for emptying of the synaptic vesicles and in inactive stage binds many vesicles prepared for exocytosis.
Synaptic vesicles contain a quantum of neurotransmitter. The quantum is a minimal amount which can be released by presynaptic terminal. The number of released quanta depends on the frequency and number of incoming action potentials.
Mitochondria create ATP necessary for the synthesis of neurotransmitters.
Mechanism of neurotransmitter quantum releasing
There is high density of voltage gated calcium channels in the membranes of presynaptic terminals.
Calcium channels are opened when the action potential depolarizes membrane and then calcium cations start to diffuse to the cytoplasmic fluid of the terminal according to their concentration gradient. The number of emptied synaptic vesicles (quanta) is in direct proportion to the quantity of opened calcium channels.
Calcium ions bind to the proteins of active zone. These special proteins are classified to the superfamily SNARE and we know about many of them. We find them in the membrane of active zone and in the membrane of synaptic vesicles. They change their conformation after binding of calcium ions and SNARE proteins of the vesicle and active zone are going to interweave. So they will squeeze together the membrane of the vesicle and membrane of the presynaptic terminal thus finally merge them and the quantum of neurotransmitter is released to the synaptic cleft. This mechanism is called zip theory.
In addition, there are receptors on the presynaptic membrane called autoreceptors which react to own neurons released neurotransmitters. These proteins have regulation function and monitor concentration of the neurotransmitter in the synaptic cleft.
Besides the monitoring of the own neurotransmitter is presynaptic terminal influenced by chemical substances from other neurons, they are paracellular signals or neurotransmitters secreted by other neurons to the neighbourhood of the cleft. They activate so called heteroreceptors which also change the activity of presynaptic terminal.
Receptor protein has to affect metabolism of another substance than own ligand to be considered as a heteroreceptor.
Postsynaptic terminal
By postsynaptic terminal is meant the part of postsynaptic neuron which neighbours with the presynaptic terminal and where are exprimed specific receptors in very high density on own cell surface.
We describe two very important structures at terminal: postsynaptic density and receptor proteins.
Postsynaptic density
Postsynaptic density is dense net of fibrous proteins located inside of the cell closely in the near of plasmatic membrane of the terminal. It has many functions:
1) Maintains receptor proteins in appropriate position
It is necessary to anchor molecules of those proteins in relatively small piece of membrane to keep high density of receptors. Membrane has a fluidal character and receptor proteins would travel spontaneously unanchored and because of this It will be impossible to maintain their high density.
2) Regulates number of exposed receptors
It can withdraw receptor proteins from membrane because of wearing out or just excess (internalization of receptor). Postsynaptic density can also expose new receptors on membrane, these are often prepared in vesicles bound here.
3) Changes morphology of synapse
Synapse can change shape thanks to postsynaptic density in some cases. Some parts of CNS are creating specialized processes after frequent and long term stimulation. So called dendritic spines are changing characteristics of synapse and we believe that they are involved in creating memory pathways. These structures are able to rearrange, appear or disappear in a minute. Some poetic authors describe them dancing. It is complicated process showing CNS as very dynamic system capable of quick changes. Characteristics of postsynaptic density allow forming of new connections between neurons and destroying of old unemployed.
Receptors
Receptors are proteins incorporated in cytoplasmic membrane carrying binding sites for neurotransmitters. Receptor is activated by interaction with neurotransmitter and so signal is conducted. This switch is off three type in common:
1) Ion channel linked
2) G-protein linked
3) Enzyme linked
1) Ion channel linked
The aim is usually to change membrane potential of the target cell when is receptor linked with ion channel. An open channel allows migration of charge across the membrane. This change is prompt and leads to activation of voltage gated channels. Activity of channels – both receptor associated and voltage gated – changes permeability of membrane and chiefly changes ion contain in its neighbour. Character of this change depends on if the activated channel is permeable for cations or anions.
Cation channels are according to their diameter and other attributes permeable for sodium, potassium or calcium. Their orifice is of negative charge what is attracting positively charged ions and keeps away anions.
Anion channels are permeable mostly to chlorides. Hydrated ions of sodium, potassium and calcium are too big and so can’t go through, because of this the most of chloride channels don’t have to be of negative charge.
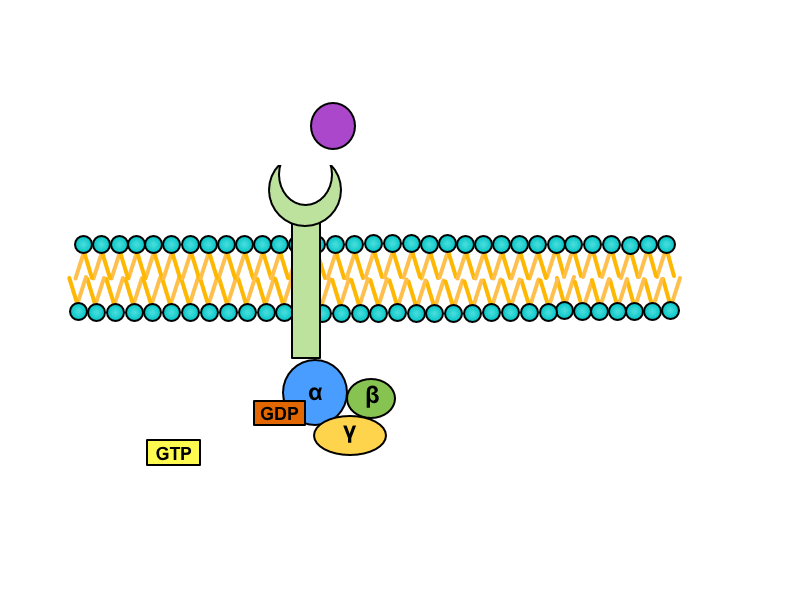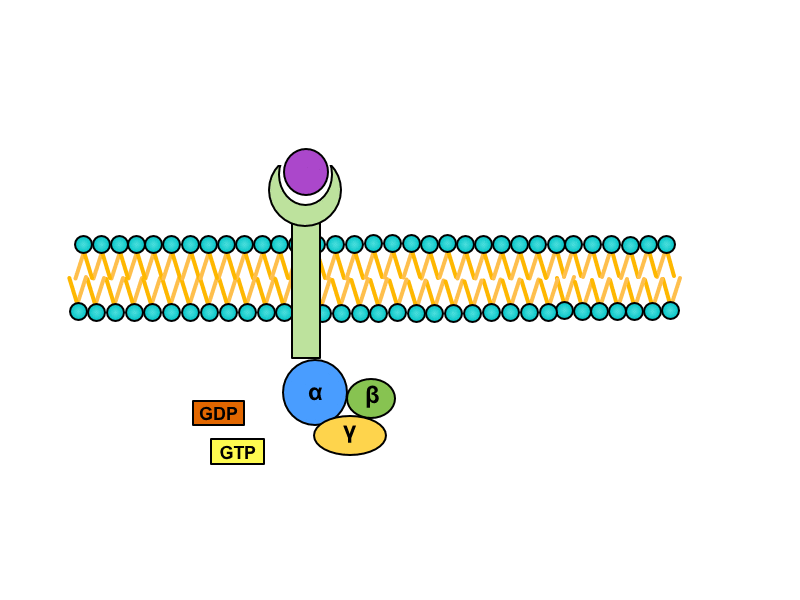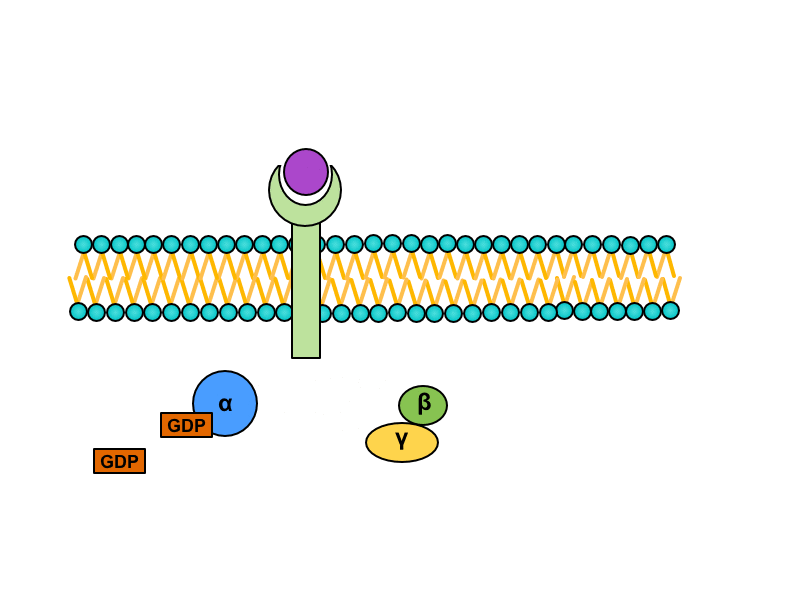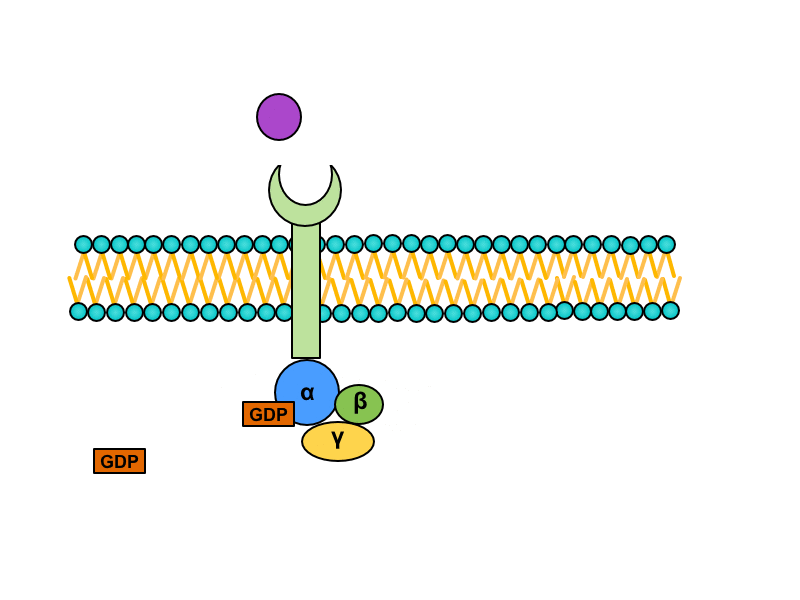
2) G-protein linked
Some function (e.g. learning and memory) of CNS require long time changes remaining even after the end of the neurotransmitter-receptor complex. The activation of ion channels lasts a short time and their effect disappears in milliseconds after the deactivation. Linking of signal to second messengers can last even months, no matter if using G-protein or intracellular enzyme.
Serpentine receptors with transmembrane domain crossing plasmatic membrane seven times are coupled with so called G-proteins. They are a heterotrimeric GTP binding proteins whose are associated with receptors in inactive stadium. One G protein is composed of subunits alpha, beta and gamma. GDP is bound to subunit alpha. Activation of the serpentine receptor launches conformational change of G-protein, GDP is released from the subunit alpha and its place is taken by GTP. This change leads to dissociation of heterotrimer. Result of this dissociation is monomer alfa and heterodimer beta/gamma. Both products diffuse along the membrane and connects to enzymes on which are binding sites for them.
There are many Final effects as activation of enzymes modifying postsynaptic density or changes in expression of genome modulating the enzymatic equipment or the structure of cell and others.
We distinguish three important types of G-proteins:
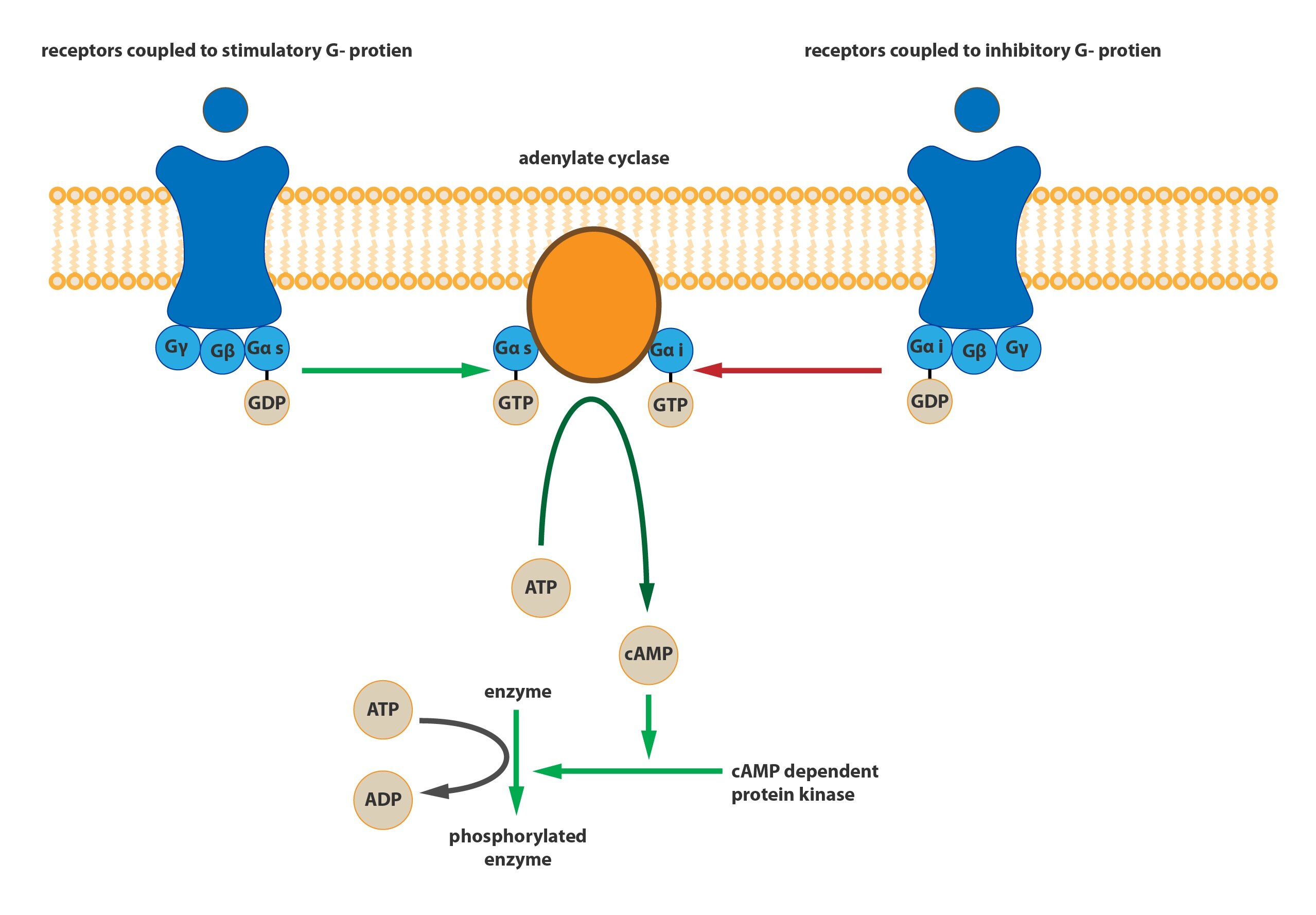
a) Gs-protein
Activates adenylate cyclase which increases concentration of cAMP. This increased concentration of cAMP in cytosol changes activity of cAMP-dependent protein kinases and finally starts variable signal transmitting cascades in cooperation with expression in an each neuron.
b) Gi-protein
Inactivates adenylate cyclase and so lowers the concentration of cAMP in cells.
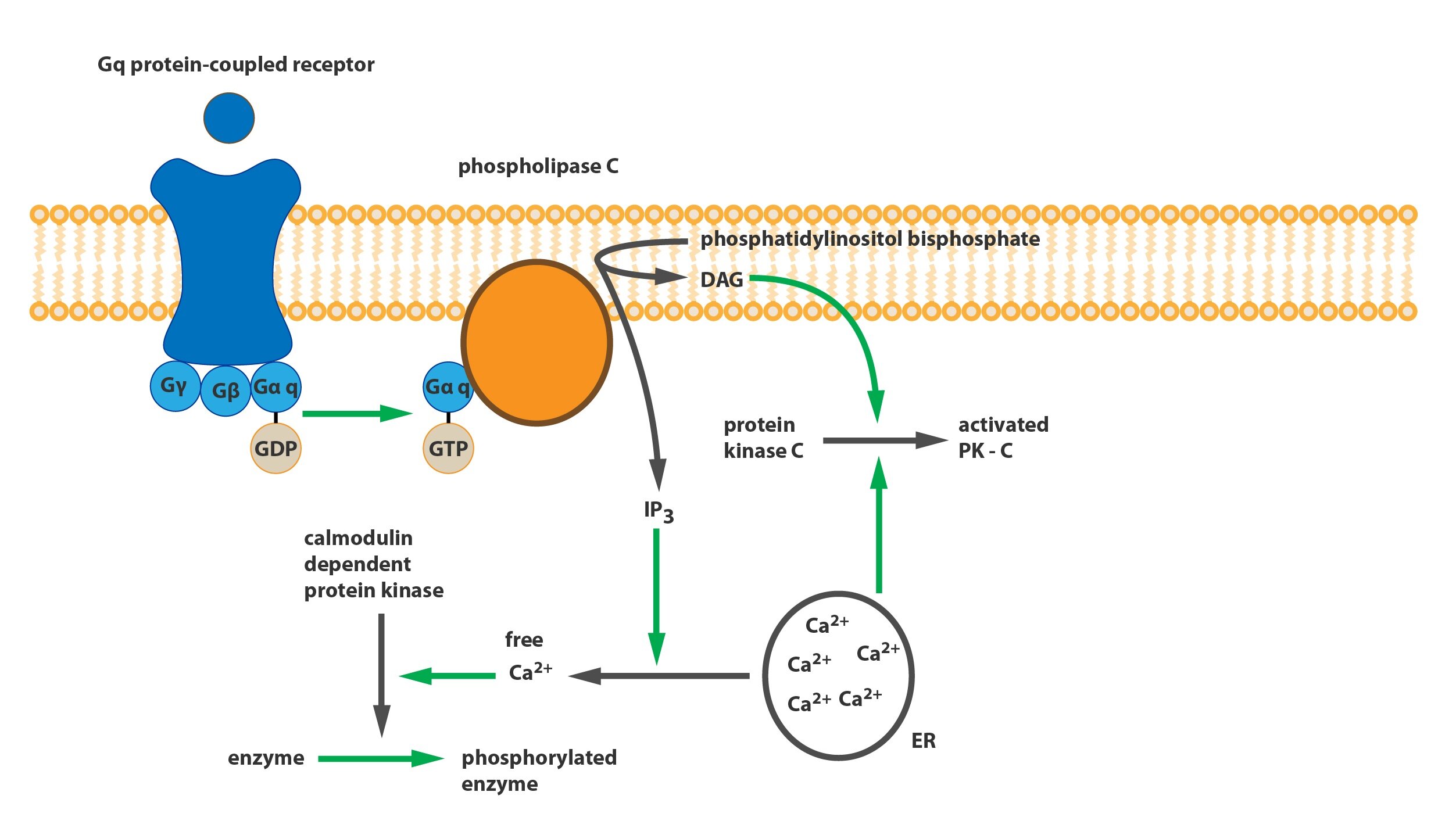
c) Gq-protein
It modulates phospholipase C (PLC). This phospholipase is catalyzing the breakdown of phosphatidylinositol bisphosphate (PIP2) incorporated in a cell membrane to inositol triphosphate (IP3) and diacylglycerol (DAG). The IP3 is involved in opening calcium channels of endoplasmic reticulum (and of mitochondria) and so calcium ions can diffuse to the plasmatic space and triggers calcium-calmodulin cascade (activation of calmodulin-dependent protein kinases). DAG with mobilized calcium activates protein kinase C (PKC).
Inactivation of G-protein
Subunit alpha has an GTPase activity, thanks to this splits bound GTP to GDP and inorganic phosphate. This change leads to reassociation of subunits alpha and beta-gama to the same form of the original trimer linked to the receptor.
3) Enzyme linked
In is very similar to G-protein linked receptor, but they differ in an initial activation of intracellular cascade. Receptors can be coupled with enzymes in two ways: receptor is associated with an enzyme molecule (and receptor activation activates the enzyme also) or receptor itself works as an enzyme.
Excitation and inhibition induced by receptors
Strict regulation of concrete systems is necessary for reactions of the CNS to changes in environment. Basal regulation is reached by increase in the activity (excitation) or it’s reduction (inhibition) on the contrary.
Excitation
Molecular mechanisms leading to excitation are:
1) Opening of sodium channels
Opening of sodium channels allows the migration of large amount of positive charge inwards the postsynaptic neuron. This charge leads to change in the membrane potential. Action potential is provoked when this change is sufficient to reach the threshold. For the reaching of the threshold is needed activation of many postsynaptic terminals in one moment (space summation) or multiple activation of just one terminal with some time interval between (time summation). Activation of sodium channels is the most common mechanism leading to excitation used in regulation of the CNS.
2) Closing of potassium channels
Inhibition of the efflux of the positively charged potassium ions is the base for accumulation of positive charge inside of the cytoplasm of the postsynaptic neuron. Membrane potential is therefore becoming more positive and is coming up to the threshold.
3) Closing of chloride channels
Impedes the influx of negative charge (of chloride ions) to the cytoplasm of the neuron and leads to the same change as the retention of potassium. Membrane potential is rising again, it is excitation.
4) Changes at postsynaptic density
They can be induced by activation of some enzymes or change in genetic expression. Changes leading to the creating of more excitatory receptors or to the reduction in the number of inhibitory receptors are considered as excitatory.
Inhibition
Has these molecular mechanisms taking place at cell membrane:
1) Opening of chloride channels
Opening of chloride channels leads to quick influx of negatively charged chloride ions into the cytoplasm of postsynaptic neuron so to decrease in the membrane potential and to rise the distance from threshold value.
2) Opening of potassium channels
Augmentation of permeability of the cell membrane for potassium allows efflux of cations out of the cell. Because of that is the membrane potential reaching more negative values.
3) Changes at postsynaptic density
They are dependent on different enzymes as are used in excitatory modifications. When are provoked inhibitory changes at postsynaptic density there is an increase in the number of inhibitory receptors or excitatory receptors are internalised.
Uniform distribution of electrical changes
Perikarion of the neural cell has relatively large diameter and is full of electrolyte fluid (intracellular fluid). According to this is perikarion minimal resistance for the conducting of electrical current so every change even on a small part of the membrane manifests in the whole perikarion. This principle is very important for the summation of changes of the membrane potential and differs from the conducting on dendrites (see below).
Excitatory postsynaptic potentials (EPSPs)
When excitatory receptors at the postsynaptic terminal become activated the change in the membrane potential doesn’t suffice to develop the action potential in the most of those cases.
This change is usually just partial. For example from -65 mV just to -60 mV, so excess of the positive charge made by excitatory impact neutralize just the piece of the negative charge of the membrane potential. This change to the more positive values is called excitatory postsynaptic potential – EPSP. In the mentioned case had EPSP value of +5mV.
The action potential is provoked in the case when the EPSP reaches the threshold. The start of the action potential is not at the site where the change was developed, action potential begins at initial segment of axon.
Changes in the membrane potential manifest in the whole perikaryon thanks to the fact, that the soma of neuron is a perfect conductor. So the initial segment receives this change and it’s membrane is modified to generate action potential effortlessly, in the location of the initial segment has the plasmalemma many more sodium channels than the rest of the cell membrane. Because of that is initial segment able to generate the action potential when the value of the change made by EPSPs is between +10 to +20 mV.
The provoked action potential travels from the initial segment in both directions: to the ending of the axon where causes a release of the transmitter quanta and back to the body of neuron, here it fades away at dendrites.
It is wrong to think that the retrograde direction of the action potential is purposeless. Action potential develops here tabula rasa effect. Changes of ion concentration related to the action potential are restoring ion concentrations to the former condition and so gets dendrites ready for another interneuronal signal transmitting.
Inhibitory postsynaptic potentials (IPSPs)
Resting membrane potential of α-motoneuron of spinal cord is approximately -65 mV. Nernst potential for chloride ions is -70 mV and therefore membrane potential is lowering after the opening of chloride channels. This decrease in membrane potential can achieve a maximal value of -70 mV and another open chloride channels will never decrease the measured potential to some more negative value, because chloride ions can’t diffuse into the neuron according to their gradient. Achieved the value of -70 mV, it is established dynamic equilibrium.
On the other hand the Nernst potential of potassium extends at the more negative value of -86 mV. So opening of potassium channels can easily hyperpolarize the membrane of postsynaptic terminal. Every influence changing the membrane potential to more negative value is called inhibitory postsynaptic potential (IPSP). For example the change from -65 mV to -70 mV is caused by IPSP of -5 mV.
This type of inhibition is called postsynaptic inhibition and is evoked by hyperpolarization of postsynaptic neuron by IPSPs.
Presynaptic inhibition
There is just one type of the inhibition during which is the activity of the presynaptic neuron changed before the exocytosis of vesicles to the synaptic cleft.
The principle of this type of inhibition is in the impact of the inhibitory transmitter exocytosed to the neighbourhood of the axon of the presynaptic neuron. This transmitter opens chloride channels of axon so hyperpolarizes the membrane of the nodes of Ranvier an inhibits the propagation of the action potential to the axon ending.
The most common type of this transmitter is GABA. Its effect is utilized in sensory pathways where fibers are inhibiting each other to increase an accuracy of stimulus, for example the high of tone in hearing.
Space summation
As mentioned the only one EPSP is insufficient to generate the action potential at initial segment.
There is stimulated big group of postsynaptic channels at different places of the perikaryon in the same time ordinary. Perikaryon is almost ideal conductor and every EPSP is counted up. This phenomenon is called a space summation.
Time summation
When the postsynaptic neuron is influenced by new EPSP it makes ion changes lasting up to 15 ms after the closing of the responsible channel. If it’s stimulated in this interval the same place as before it leads to adding of the ion changes. Repeated discharges of one presynaptic terminal are counted up if there is interval no longer than 15 ms. This phenomenon is called time summation
We can subtract EPSPs form IPSPs or add the effect of IPSPs in the same principle.
Facilitation
When nor summated EPSP isn’t sufficient to reach the threshold its value is decreasing but its value is remaining near the threshold than in naive state for a while. Another excitatory impulse arrived into this neuron can in the moment provoke the action potential despite the fact that the value of this only one EPSP can’t induce the action potential alone. This phenomenon is called facilitation. Facilitated neuron can generate action potential if is stimulated even by subliminal impulse.
Dendrites
Dendrites are highly specialized structures of neurons and they are adapted to accept signals from presynaptic terminals. We have been talking about various phenomena ongoing at the body of the neural cell until now. There are significant differences between dendrites and the perikaryon.
Dendrites can’t generate the action potential because they have relatively small amount of sodium channels. Dendrites are just able of electric conducting – just conducting of ion current – to the body of the neural cell. Thanks to their small diameter put up higher resistance to this current. They aren’t ideal conductor absolutely in contrast to the body. Their cell membrane is thin and permeable for ions. Because of those facts there are losses in conducting, these losses are bigger according to higher distance of EPSP to soma.
For example: EPSP with value of +5mV can be totally lost when conducted to the perikaryon and so doesn’t induce any effective change at the initial segment of axon. Conducting with losses is called decremental conduction – is typical for dendrites. EPSPs developed somewhere on the soma are conducted without decrement.
As mentioned, decrement rises with a higher distance from the perikaryon. So synapses situated at the margin of dendrites and the soma are more effective than synapses far away. Their effect will be (however excitative or inhibitory) much greater.
Powerful excitatory stimulus located at the terminal arborization can be completely denied by some only weak inhibitory stimulus. This phenomenon impedes summation very much.
Fatigue of synaptic transmission
If we will stimulate presynaptic terminal many times with high frequency the number of discharge of the postsynaptic neuron is very high at start but it progressively decrease after few seconds. This phenomenon is called fatigue of synaptic transmission.
The mechanism of this fatigue is depletion of neurotransmitter quanta, because they suffice for only about 10 thousand of action potentials. Another factor is advancing inactivation of receptors of the postsynaptic terminal and development of abnormal ion concentration in the cytoplasm of the postsynaptic neuron.
The point of this fatigue of synapse is in protection of systems of the CNS against the excessive activity of neural cells. The most evident significance we can observe at a generalised tonic-clonic seizure which ends thanks to this mechanism of fatigue of the epileptogenic centre.
Subchapter Authors: Patrik Maďa and Josef Fontana