Content:
1. Introduction to neurotransmission systems
2. Glutamatergic system
3. GABAergic system
4. Cholinergic system
5. Catecholamines as neurotransmitters
6. Serotonergic system
7. Glycinergic system
8. Histaminergic system
9. Purinergic neurotransmitters
10. Peptides
11. Endocannabinoids
_
Introduction to neurotransmission systems
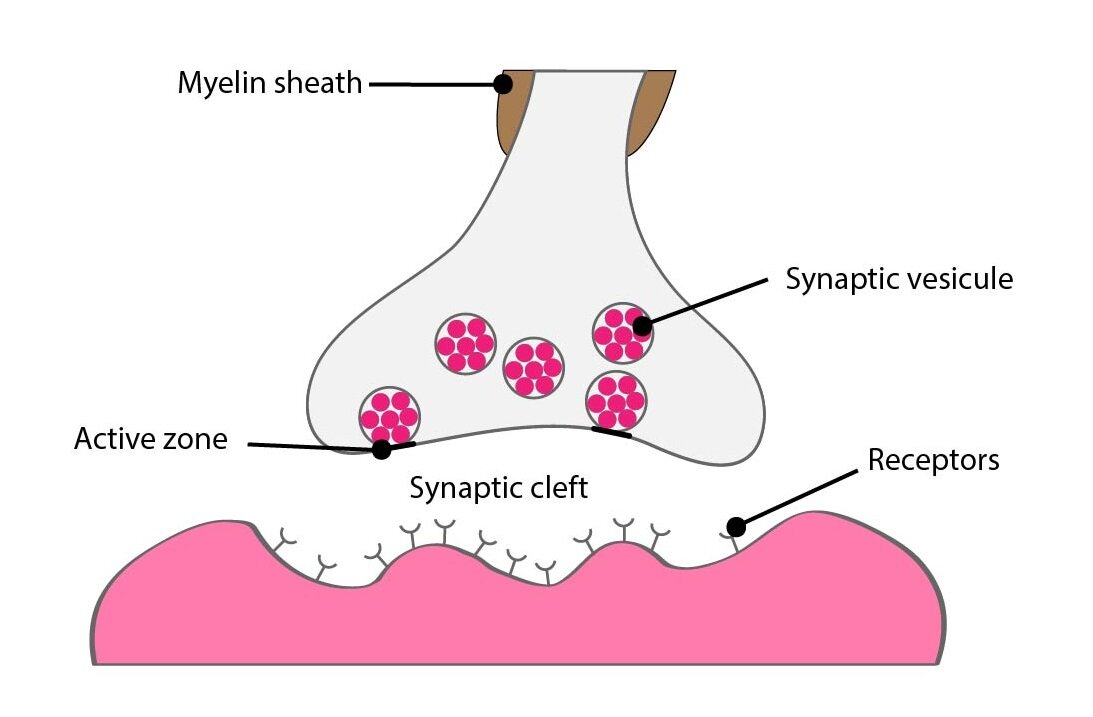
Neurotransmitter is a substance released by the neuron to the concrete target cell (or cells) where induces specific response. This target can be another neural cell or organs, especially glands and muscles.
In contrast to the endocrine signalisation is neurotransmission targeted to the cells in the close neighbourhood of the neuron releasing the substance to the synaptic cleft or just to the vicinity of so called varicosities (buttons en passant) in the autonomic nervous system.
Difference between neurotransmission and paracrine signalisation is that neurotransmitter doesn’t effects only to target cells but to the releasing neuron too. This effect usually modulates another neurotransmitter releasing or the synthesis of neurotransmitter itself.
Another important attribute is that neurotransmitters binding to receptor is just temporary and lasts from just a few milliseconds to minutes. In the other hand provoked changes dependent on the type of transmission can last for while too or persist for days or even weeks.
Immediate impact are usually caused by ionotropic (working by the influence of the ions) receptors effecting as ion channels (or are linked to them) and leads to the change of the membrane potential and maybe to the threshold of depolarisation and to elicit the action potential.
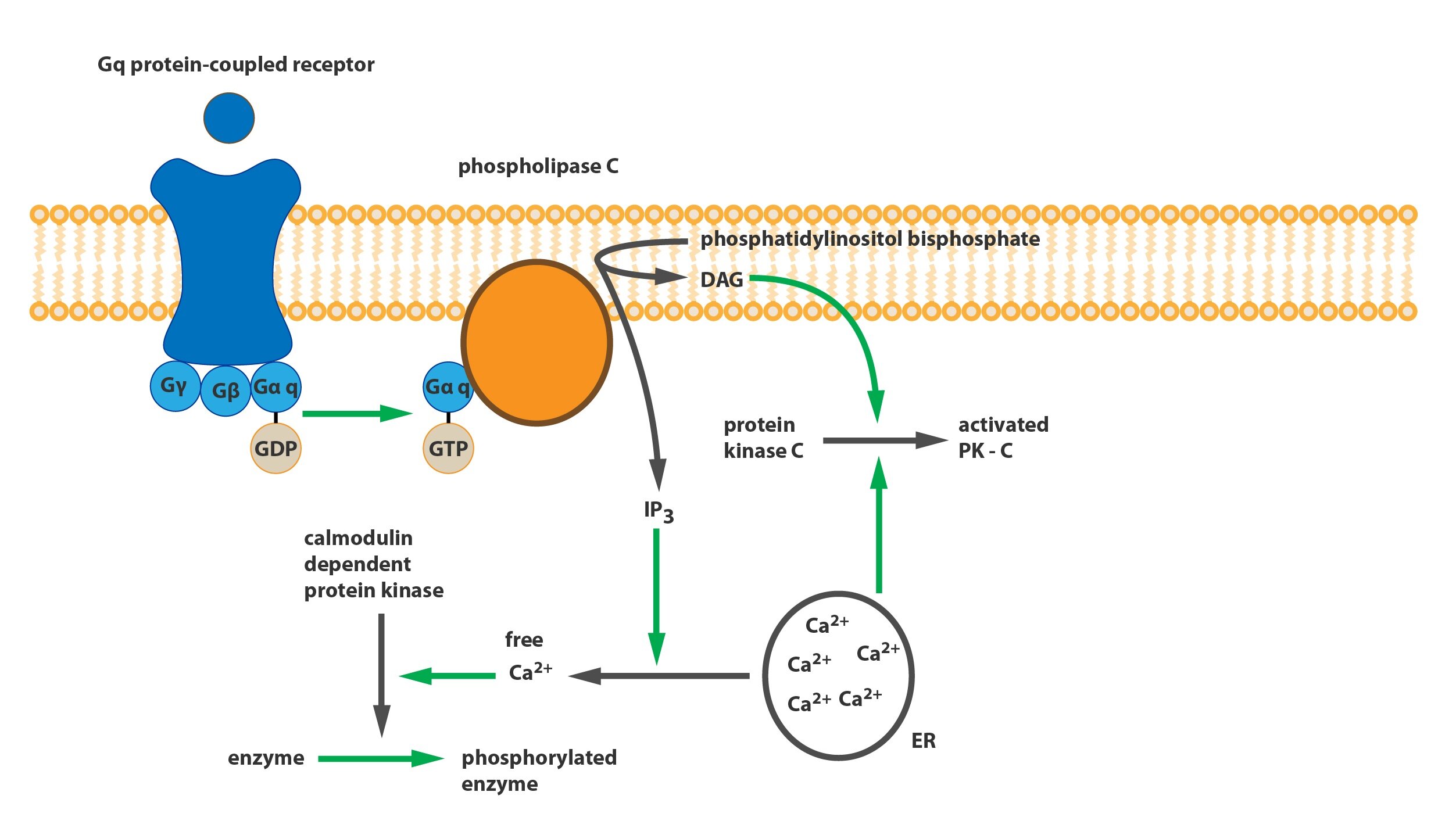
Long term changes of the target cells are mediated by the bond of the neurotransmitter to the metabotropic receptor which is often activating intracellular signal cascades via G protein. These continuing pathways cause change in the expression of various genes or activation of preexisting enzymes (e.g. phosphorylation/dephosphorylation cycle).
The concept of neurotransmission as the only intercellular communication of a one neuron to another one or to some organ is not completely right. It shows, that some glial cells, astrocytes in the most, are able to synthesize the neurotransmitter and are able to release it to their target cells as they are able to exprime according receptors on their membrane.
There exist a wide range of substances that we can find in the nervous system and which are synthesized by neurons. Only some we can consider as neurotransmitters so fitting to these four criteria:
1) Substance is synthesized by presynaptic neuron
2) Substance is stored in the presynaptic ending and released in sufficient amount to elicit the change in the postsynaptic neuron or effector organ
3) If the substance is administered by exogenic way the reaction to its presence provokes the same reaction as its endogenous release
4) The specific mechanism which inhibits of this effective substance exists
By the chemical point of view neurotransmitters aren’t unified group of substances, their structure is very variable. Basicly we can divide them to two large groups:
1) Big molecules
a) Peptides: beta-endorphins, leu-enkephalins, substance P
b) Endogenous cannabinoids
2) Small molecules
a) Amino acids: glutamate, aspartate or glycine
b) Amino acids derivatives: GABA, catecholamines – noradrenaline and dopamine, serotonine
c) Acetylcholine
d) Other: purines (ATP, ADP, adenosine), gases (NO)
In general we can say, that only precursors of neurotransmitters are crossing the blood-brain barrier to the central nervous system and not whole neurotransmitters. This information had a big influence to a treatment of some of neurologic diseases.
Synthesis of the majority of neurotransmitters takes place at the synaptic ending. But peptides builded in the soma are important exceptions.
_
Glutamatergic system
Glutamate is the main excitatory neurotransmitter in the CNS. It is distributed so widely that it is impossible to talk about individual centres or projections.
It plays an indispensable role in synaptic plasticity, i.e. the removal of old, unused synapses, potentiation of others and the formation of new synapses. These changes, to a large extent mediated by the glutamatergic system (not exclusively), may in principle alter interneuronal connections virtually on a minute by minute scale.
It is thought that synaptic plasticity is the neurobiological process behind memory retention and learning.
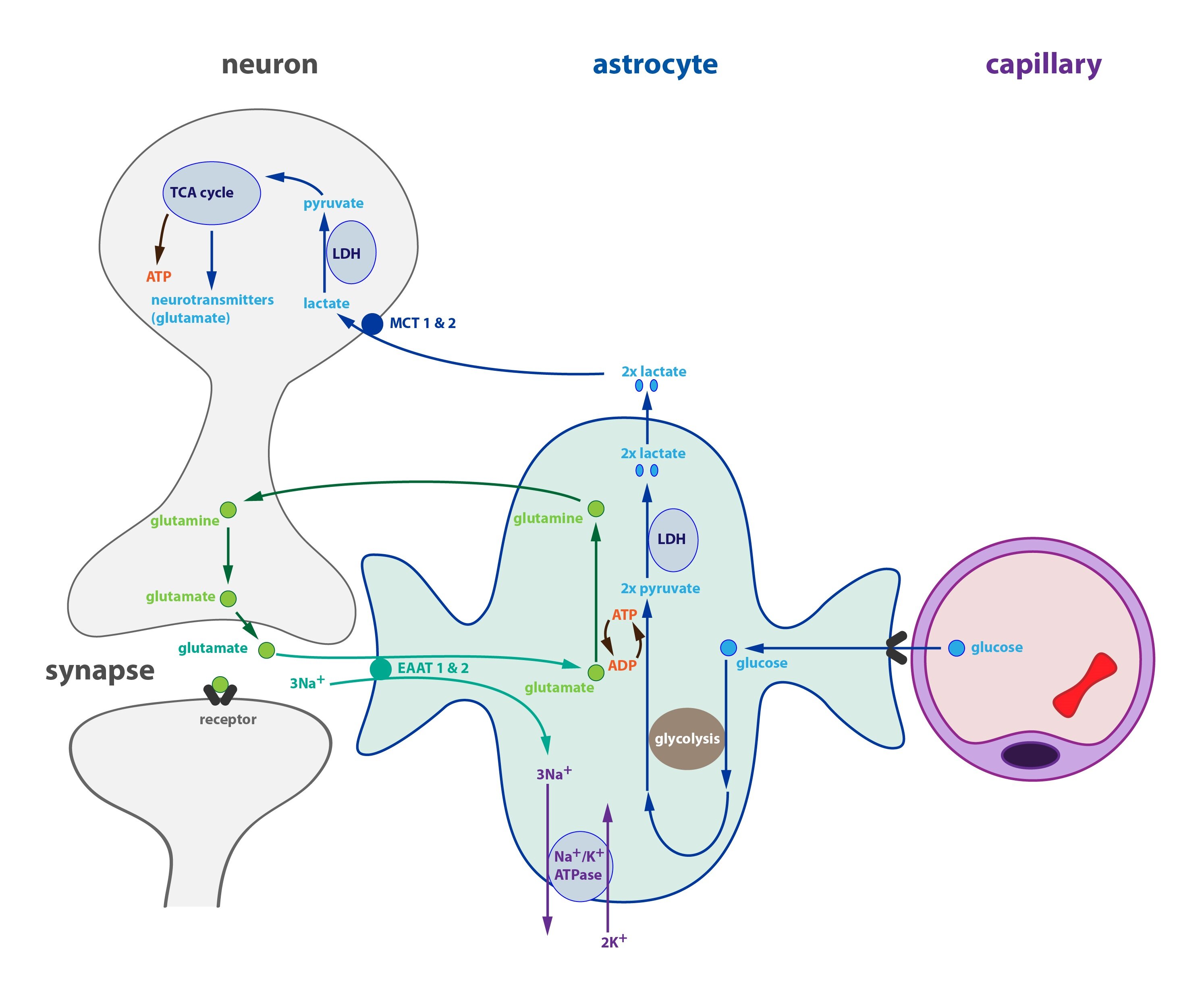
Synthesis and inactivation of glutamate – cycle neuron-astrocyte
The blood-brain barrier is set up in such a way that glutamate essentially never traverses from blood into the brain. Under normal conditions glutamate is only transported from the CNS into blood.
Most glutamate is produced in neurons from glutamine (via glutaminase), which is mainly formed in astrocytes and transported into neurons. A close cooperation between neurons and astrocytes is therefore essential for the production of glutamate and GABA.
Upon release into the synaptic cleft only a small portion of glutamate is re-uptaken into the presynaptic neuron; most of it is transported via glutamate transporters EAAT1 and EAAT2 (Excitatory Amino Acid Transporter) into astrocytes.
Glutamate is transformed into glutamine inside an astrocyte, which reaction requires ATP and ammonia and is catalysed by glutamine synthetase. Glutamine is then exported into neurons hence completing the cycle.
Clinical correlation:
Some diseases such as liver failure may cause an elevation of blood ammonia levels. Ammonia as an uncharged species easily traverses the blood-brain barrier and in the presence of glutamate it can be converted into glutamine while consuming ATP. If there is too much ammonia, neuronal ATP may be depleted and at the same time two major neurotransmitter systems will be dysregulated – glutamate and GABA. This condition is called liver encephalopathy. Elevated blood ammonia is one factor by which liver disease may influence CNS function.
One may ask why astrocytes recycle glutamate in such a complicated manner instead of handing it over to neurons directly. One possible explanation is that an uncontrolled release of glutamate into the extracellular fluid could excite neighbouring neurons. to prevent this glutamate is first converted to glutamine. Latest research shows that astrocytes can indeed release glutamate but do so in a highly controlled manner.
There are several types of glutamate receptors categorised according to their specific pharmacological agonists:
1) AMPA receptors
2) NMDA receptors
3) Kainate receptors
4) Metabotropic receptors (mGluR1-8)
1) AMPA receptors (AMPARs)
These are ligand-gated ion channels, i.e. ionotropic receptors. Their opening allows the influx of calcium and sodium and the efflux of potassium. They close soon after opening.
2) NMDA receptors (NMDARs)
These are also ionotropic receptors increasing the permeability for calcium, sodium and potassium. The opening of NMDA receptors requires in addition to a conformational change the removal of a magnesium ion, which blocks the pore. Magnesium leaves the channel only if the membrane is sufficiently depolarised (e.g. via AMPARs), which alteration of the electric field repulses the cation.
NMDARs allow a calcium influx sufficient for the activation of calcium-dependent enzymes, which can then regulate properties of the postsynaptic density, receptor density in the membrane, etc. These processes are very important for synaptic plasticity.
3) Kainate receptors
Kainate receptors are also ionotropic and functionally similar to AMPARs.
4) Metabotropic receptors
There are 8 known types of metabotropic receptors labelled as mGluR1-8. They are fond, e.g. on presynaptic neurons, where they attenuate their activity or increase the turnover of phosphatidylinositol.
_
GABAergic system
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the CNS. Similar to glutamate its distribution is diffuse and a distinct neurotransmitter system cannot be identified.
Synthesis and inactivation of GABA – cycle neuron-astrocyte
Most GABA is synthesised by the decarboxylation of glutamate catalysed by glutamate decarboxylase. As with glutamate, the precursor is glutamine transported from astrocytes. Glutamine is converted inside a neuron to glutamate (catalysed by glutaminase), which is then decarboxylated to GABA.
Once released into the synaptic cleft GABA is mainly taken up by astrocytes and converted back to glutamine. The gamma-amino group of GABA is first transaminated to give an aldehyde group thus forming succinate semialdehyde. This reaction is catalysed by GABA transaminase. Succinate semialdehyde is then oxidised to succinate, which enters the TCA cycle. A half-turn of the cycle takes us to 2-oxoglutarate, which can be (trans)aminated to glutamate and further amidated to glutamine using ammonia and ATP. Glutamine is then exported and the cycle closes.
There are three subtypes of GABAergic receptors:
1) GABAA
This is an ionotropic receptor allowing the permeation of chloride anions. Its opening generally leads to an influx of chloride into the cytoplasms and hence to a lowering of the membrane potential.
It forms a supramolecular receptor complex, which in addition to GABA binds benzodiazepines, barbiturates, corticosteroids and alcohol. All these binding sites affect the opening of the central pore and all act to inhibit the neuron either directly via opening the pore or indirectly by potentiating the binding of GABA.
2) GABAB
This is a metabotropic receptor couple to a Gi protein inhibiting adenylate cyclase. The resulting decrease in cAMP levels changes membrane protein phosphorylation status, which leads to an increase in potassium permeability (membrane hyperpolarisation) and a decreased activity of calcium channels. The result is a decreased amount of neurotransmitter released by the neuron.
This receptor is found in abundance in many areas of the cortex, thalamus and cerebellum.
3) GABAC (recently renamed to GABAA-rho receptor)
This is an ionotropic receptor connected to a chloride channel. In contrast to GABAA receptors they open more slowly and remain open for a longer period of time.
_
Cholinergic system
Acetylcholine is the only neurotransmitter containing a quaternary ammonium group.
It is the neurotransmitter used in neuromuscular junctions of all vertebrates, ale preganglionic neurons of the autonomous nervous system and all postganglionic parasympathetic neurons. In the CNS it modulates many cortical activities such as arousal, sleep and memory consolidation.
Synthesis and inactivation of acetylcholine
Acetylcholine (ACh) is an ester of choline and acetic acid. It is synthesised in one step by transferring the acetyl group from acetyl-CoA to choline. This reaction is catalysed by choline acetyltransferase.
The brain receives all its choline from the blood. Choline is mostly synthesised in the liver by a triple methylation of ethanolamine; S-adenosylmethionine (SAM) is the donor of methyl groups. Ethanolamine is produced by a decarboxylation of serine.
The cholinergic signal is terminated by the serine hydrolase acetylcholine esterase (AChE) bound to the postsynaptic membrane. The hydrolysis produces choline, which is taken up by the presynaptic neuron and recycled, and acetate. The catalytic activity of AChE depends primarily on three amino acid residues – serine, histidine and glutamate (a similar triad exists also in serine proteases with aspartate instead of glutamate). The hydroxyl group of serine attacks the ester bond. AChE is not entirely specific for ACh, it also hydrolyses other choline esters.
Cholinergic signalling in the nervous system is mediate by two receptor types with several subtypes:
1) Muscarinic receptors
These are metabotropic receptors coupled to G proteins, which then regulate ion channel opening. The response of the postsynaptic neuron is thus relatively slow. So far five subtypes of muscarinic receptors have been identified.
a) M1 receptors
These so called neuronal receptors are found in abundance in the CNS, particularly in the hippocampus and cortex. This receptor mediates an excitatory response via a Gq protein starting a signalling cascade leading to a decreased permeability for potassium. It is thought that a decrease in their function or density is one of the causes of dementia.
b) M2 receptors
Often labelled as cardiac receptors they are expressed in cardiomyocytes but can be found in high densities in neuronal tissues as well. They mediate an inhibitory response via a Gi protein, which activates potassium channels (via its beta-gamma subunit dimer) thus causing membrane hyperpolarization. This is the mechanism by which the vagus nerve exerts its negative chronotropic effect on the sinoatrial node and the negative dromotropic effect on the atrioventricular node. In the CNS M2 receptors act as autoreceptors on presynaptic neurons mediating negative feedback inhibition in the cortex and the hippocampal formation.
c) M3 receptors
These receptors mediate the cholinergic stimulation of exocrine glands and the contraction of smooth muscles in the GIT and other organs. M3 receptors are coupled with a Gq protein, which increases intracellular calcium concentration via the activation of phospholipase C and the formation of IP3 and DAG from PIP2. Although they exist in the CNS at a relatively low density they can induce a potent emetic effect.
The effect of ACh on blood vessels is worth pointing out. While ACh can cause smooth muscle contraction in blood vessels it causes vasodilation. This is not a direct effect on the smooth muscle but rather a heterotropic inhibition of noradrenergic sympathetic activity and the stimulation of NO production in the endothelium.
M3 receptors are being intensively studied due to their role in the development of type 2 diabetes. In the CNS they are expressed in areas responsible for the monitoring and regulation of blood glucose levels, i.e. in parts of hypothalamus and parasympathetic nuclei of the brainstem. In the pancreas they are expressed in beta-cells of the islets of Langerhans, which explains why certain antipsychotic agents used to treat schizophrenia may cause blood glucose dysregulation and increase the risk of T2D via blocking M3 receptors.
d) M4 receptors
This group of receptors is still relatively poorly studied. Their mechanisms of effect is similar to M2 receptors (Gi-protein activating K+ channels). They are found mainly in the striatum, where they function mainly as regulatory autoreceptors on cholinergic neurons (the same role as M2 receptors in the hippocampus and other cortical regions).
In the striatum M4 receptors attenuate the activity of excitatory D1 receptors, through which dopamine increases the activity of the extrapyramidal motor system. The physiological implications of this interaction are unclear.
e) M5 receptors
Similar to M1 and M3 receptors M5 receptors are couple with a Gq-protein. Most studies on M5 receptors to date were performed in vitro.
2) Nicotinic receptors
These are ionotropic receptors opening cation channel permeable for sodium and potassium, in some subtypes also for calcium. The basic division is into a muscular type (NM receptor) present mostly in the neuromuscular junction and a neuronal type (NM) found in all postsynaptic terminals in autonomic ganglia. In the CNS NM receptors function as heteroreceptors for other systems (GABA, serotonin, glutamate, dopamine). They increase the permeability for calcium and increase the amount of released neurotransmitters.
Cholinergic neurons are found mainly in the basal nucleus of Meynert and in septal nuclei.
These neurons project into the cortex and the hippocampus. They play a role in the activation of certain cortical areas and in short term memory consolidation. The neurons of the basal nucleus and medial septum are damaged in Alzheimer’s disease.
Another group of cholinergic neurons can be found in the tegmentum of the brainstem, which send their projections into the cerebellum, hypothalamus and lower portions of the CNS. These affect arousal, sleep cycle and are important for the initiation of the REM sleep phase.
There are cholinergic interneurons in the striatum, which form a part of the basal ganglia circuit and thus play a role in the regulation of posture, movement initiation and selection of appropriate movement patterns.
Clinical correlation:
Some acetylcholine esterase inhibitors are used to treat diseases such as Alzheimer’s disease or myasthenia gravis. Some are highly toxic substances such as extremely effective organophosphates. These compounds form a strong covalent bond with the OH group of the serine residue in the active site of the enzyme, which lasts for weeks. Organophosphates are mostly used as insecticides and as such can cause accidental intoxications. There are also highly toxic volatile organophosphates used as nerve gases such as sarin, tabun and VX.
_
Catecholamines as neurotransmitters
Catecholamines (noradrenaline, adrenaline and dopamine) are important neurotransmitters in the CNS. In this chapter we will mostly cover the functions of the noradrenergic and dopaminergic systems.
Synthesis and inactivation of catecholamines
All catecholamines are derived from the aromatic amino acid L-tyrosine (or from phenylalanine via tyrosine). Their structure contains a catechol ring – benzene ring with two hydroxyl groups – with a side chain containing an amino group.
The conversion of tyrosine to adrenaline occurs in the following steps:
1) Ring hydroxylation
2) Decarboxylation – formation of dopamine
3) Side chain hydroxylation – formation of noradrenaline
4) N-methylation – formation of adrenaline
1) Tyrosine hydroxylation
In the first step L-tyrosine is converted to L-dihydroxyphenylalanine (L-DOPA). The reaction is catalysed by tyrosine hydroxylase, the rate limiting enzyme for catecholamine synthesis. The main regulatory mechanism is feedback inhibition by catecholamines.
2) DOPA decarboxylation
In the second step L-DOPA is decarboxylated to the biogenic amine 3,4-dihydroxyphenylethylamine – dopamine. This reaction is catalysed by DOPA decarboxylase a.k.a. aromatic L-amino acid decarboxylase (LAAD) as it can decarboxylate other aromatic amino acids (see also serotonin synthesis).
Clinical correlations:
Alpha-methyldopa, is a competitive inhibitor of DOPA decarboxylase and is used to treat hypertension.
Catecholamines are unable to cross the blood-brain barrier (BBB) and must be synthesised directly in the CNS. Parkinson’s disease is characterised by an insufficient formation of dopamine in the brain and L-DOPA as a dopamine precursor can be used for its treatment because it can cross the BBB.
3) Dopamine hydroxylation
The third step is the hydroxylation of the dopamine side chain and the formation of noradrenaline. This reaction is catalysed by dopamine beta-hydroxylase.
4) Noradrenaline N-methylation
The last step is the methylation of the noradrenaline amino group and the formation of adrenaline. This reaction is catalysed by phenylethanolamine-N-methyltransferase (PNMT) with S-adenosylmethionine as its co-substrate and methyl group donor. The activity of this enzyme is positively regulated by glucocorticoids (cortisol) formed in the adrenal cortex and transported into the medulla via the suprarenal portal system.
The catecholamine signal is terminated by the re-uptake of the neurotransmitter and subsequent intracellular inactivation. The two enzymes catalysing this inactivation are:
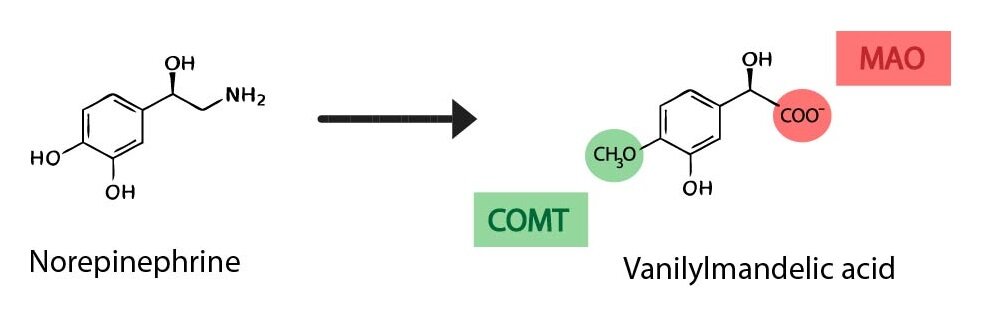
1) Catechol-O-methyltransferase (COMT)
catalyses the transfer of a methyl group from S-adenosylmethionine to a catechol hydroxyl group causing the following transformations:
Noradrenaline → normetanephrine
Adrenaline → metanephrine
Dopamine → 3-methoxytyramine
2) Monoamine oxidase (MAO)
catalyses the oxidative deamination of aromatic monoamines resulting in the formation of an aldehyde group, which is subsequently oxidised by an aldehyde dehydrogenase to a carboxyl group. This sequence is responsible for the following transformations:
Metanephrine or normetanefrin → vanillylmandelic acid (VMA)
3-methoxytyramine → homovanillic acid (HVA)
MAO is a mitochondrial enzyme bound to the outer mitochondrial membrane. It is a flavoprotein – contains a covalently bound FAD. There are two types of MAO in humans, MAO A and MAO B. Both types are found in the nervous tissue, MAO A also in the liver and GIT and MAO B also in platelets.
Clinical correlation:
MAO inhibitors (MAOI) are used to treat depression.
Vanillylmandelic acid is excreted mainly in the urine and its excretion can be used to estimate the production of catecholamines in the organism. It is mainly used when an adrenal medulla tumor (pheochromocytoma) is suspected.
Let’s now have a look at individual catecholaminergic systems.
Noradrenergic system
Noradrenaline in the CNS mainly regulates the activity of other neurotransmitter systems. Noradrenergic pathways increase or decrease the excitability of target areas depending on the receptors expressed and other poorly understood mechanisms. Noradrenergic pathways can thus regulate both the excitatory function of glutamate and the inhibitory function of GABA.
Noradrenaline binds to specific receptors in the target cell’s membrane. There are four subtypes of adrenergic receptors in the CNS:
1) α1 receptor
Present in postsynaptic neurons, mediates excitatory effects of noradrenaline.
2) α2 receptor
Present mainly in presynaptic terminals and mediates inhibitory effects. Its main role is in feedback inhibition of noradrenaline release in the synapse.
3) β1 receptor
Neuronal receptor with excitatory effects.
4) β2 receptor
In the CNS mainly expressed in glial cells. Latest research shows that beta-2 receptors mediates the integration of the nervous and immune systems. The exact physiological role is being studied.
Noradrenergic neurons are found in the brainstem, particularly in the locus coeruleus (group A6), tegmentum and the reticular formation of the medulla and pons (groups A1, A2, A5, A7).
1) Locus coeruleus
Axons of neuron in the locus coeruleus project virtually to all CNS regions. This system modulates the excitability of other projection systems and regulates attention, arousal, sleep cycle and response to stress. Projections into association cortical areas influence emotional behaviour.
2) Neuronal groups of the tegmentum and reticular formation
Neurons of these groups project into the spinal cord, brainstem, hypothalamus and the limbic system, particularly into regions responsible for the regulation of visceral functions.
In the spinal cord they regulate the excitability of anterior horn motoneurons.
The reticular formation contains a smaller group of neurons releasing adrenaline with projections similar to those of locus coeruleus. It is thought that they cooperate with noradrenergic neurons in the regulation of alarm reaction and adaptation to stress.
Clinical correlation:
The catecholaminergic hypothesis aims to explain the pathogenesis of affective disorders such as depression primarily as an hypoactivity of noradrenergic projections from locus coeruleus into certain cortical areas (with a concomitant disorder of the serotonergic system).
Dopaminergic system
Dopamine in the CNS plays an important role in the regulation of motor functions, initiation of behavioural patterns and modulation of visceral functions.
Five dopamine receptors have been identified to date, which are all coupled to a G-protein regulating adenylate cyclase activity. They can be divided into two groups depending on whether they activate AC via a Gs protein (D1, D5) or inhibit it via a Gi protein (D2, D3, D4). The density of various receptor subtypes differs in different areas of the CNS, e.g. cortical motor areas are rich in D2 receptors while the limbic system mainly expresses D3 and D4 receptors. Adrenaline and noradrenaline act as partial agonists on most dopamine receptors.
Dopaminergic neurons can be found in the mesencephalon and hypothalamus. From a functional point of view several distinct dopaminergic projections are described.
1) Nigrostriatal projection
Neurons form the pars compacta of substantia nigra project mainly into the striatum, globus pallidus and putamen and as such influence the functioning of basal ganglia. They play a role in the planning and execution of cortical activities with their main effect on motor cortex.
Decreased function of this projection manifests as tremor at rest, bradykinesis, muscle stiffness and impaired posture. Damage to these neurons may be caused by idiopathic degenerative processes (Parkinson’s disease) or atherosclerotic narrowing of the blood vessels (parkinsonism).
2) Mesolimbic projections
Dopaminergic neurons from the ventral tegmentum and mesencephalic reticular formation project into nucleus accumbens, amygdala and hippocampus. They play a role in motivation, reward and punishment, instinctive behaviour and addiction. Their increased activity leads to stereotypical behavioural patterns, repeated movements, etc.
3) Mesocortical projections
Some neurons of the ventral tegmentum and mesencephalic reticular formation also project into prefrontal cortical areas. They influence planning and execution of frontal cortex activities (e.g. attention) and affect initiation of behaviour.
Mesolimbic and mesocortical projections are closely related and are the target of some antipsychotic drugs in the treatment of schizophrenia, obsessive-compulsive disorder, attention deficit/hyperactivity disorder and Tourette’s syndrome.
4) Tuberoinfundibular projections
Inhibit prolactin secretion.
5) Intrahypothalamic projection
Plays a role in modulating various visceral functions.
Clinical correlation:
It is currently thought that schizophrenia is at least partly caused by an excessive activity of the mesolimbic system and a deficient activity of the mesocortical system.
_
Serotonergic system
The serotonergic system plays a similar role in the CNS as the noradrenergic system. It regulates a range of functions and modulates the activity of other projection systems. It often innervates the same structures and functionally complements noradrenergic afferents.
Seven types of serotonin receptors have been identified to date, 5-HT1-7R, some of which are inhibitory and some excitatory. The effect of serotonin depends on their localisation and the types of receptors expressed.
Serotonergic neurons can be found in raphe nuclei of the reticular formation, which can be divided into a rostral past with ascendent projections and a caudal part with descendent projections.
1) Ascendent serotonergic system (nc. raphe dorsalis, nc. raphe pontis centralis superior)
This system projects into the cortex, some limbic structures (hippocampus, amygdala), basal ganglia, many hypothalamic nuclei and areas and some thalamic nuclei.
It is important for the regulation of emotional behaviour, hypothalamic functions and the sleep cycle. It also modulates information processing in sensory areas (e.g. visual cortex).
2) Descendent serotonergic system (nc. raphe pontis, nc. raphe magnus, nc. raphe obscurus)
Projections from these structures project mainly to more caudal parts of the CNS: brainstem, cerebellum and spinal cord.
Caudal neurons influence pain perception via projection into posterior horns of the spinal cord. An increased activity of the serotonergic system potentiates the effect of analgesics and is essential for the effect of opiates. Their projections also modulate the excitability of preganglionic neurons of the autonomous nervous system and stimulate motor neurons in the anterior horns of the spinal cord.
Clinical correlation:
Drugs in the group of specific serotonin re-uptake inhibitors (SSRI) increase the activity of the serotonergic system and are used to treat patients with unipolar depression. Unfortunately they also decrease libido and cause food intake disorders, usually hyperphagia.
Synthesis and inactivation of serotonin
Serotonin is derived from L-tryptophan, chemically it is 5-hydroxytryptamine. Its synthesis occurs in two steps:
1) Tryptophan hydroxylation
Tryptophan is hydroxylated to 5-hydroxytryptamine, the reaction is catalysed by tryptophan hydroxylase, which is the rate limiting enzyme of serotonin synthesis.
2) Decarboxylation
Decarboxylation of 5-hydroxytryptophan gives rise to 5-hydroxytryptamine, i.e. serotonin, which is thus a biogenic amine (together with all catecholamines). This reaction is catalysed by aromatic L-amino acid decarboxylase (LAAD, see catecholamine synthesis), in this case also called 5-hydroxytryptophan decarboxylase.
Serotonergic neurotransmission is terminated by the reuptake of serotonin and its subsequent intracellular metabolism. The inactivation of serotonin is catalysed by two enzymes – monoamine oxidase (MAO) and aldehyde dehydrogenase (see catecholamine inactivation).
The product of serotonin inactivation is 5-hydroxyindoleacetic acid, which is mostly excreted in urine conjugated with glucuronic acid. It is used as a diagnostic marker for neuroendocrine tumours secreting serotonin (formerly carcinoid).
Serotonin also starts another metabolic pathway leading to the hormone melatonin. The amino group of serotonin is first acetylated and the resulting N-acetylserotonin is the methylated on the indole hydroxyl group to form melatonin.
Historical note:
The name serotonin points to its discovery as the substance in the serum, which affects smooth muscle tone.
_
Glycinergic system
Glycine is an inhibitory neurotransmitter. Its effect in the CNS is mediated by an ionotropic receptor coupled to a chloride channel causing membrane hyperpolarization. It can be found at a high density in the spinal gray matter, where it is the main neurotransmitter of inhibitory interneurons.
In addition to its inhibitory effect it is also a modulator of NMDA receptor activity, where it can cause excitatory effects via facilitating the activity of the glutamatergic system.
Clinical correlation:
Strychnine is a blocker of the glycine receptor. By inhibiting inhibitory interneurons in the spinal gray matter it causes uncoordinated spread of stimulation causing muscle spasm. Strychnin has a strong bitter taste, it is one of the most bitter substances.
_
Histaminergic system
Histamine plays many important roles in the human body, as a neurotransmitter it regulates sleep, arousal and hormone secretion in the hypothalamo-pituitary system.
Structurally it is a biogenic amine formed by a decarboxylation of histidine (catalysed by histidine decarboxylase). Once released, histamine is degraded by diamine oxidase.
The effect of histamine is mediated by three subtypes of histamine receptors. They are found in the CNS and in peripheral tissues.
1) H1 receptors
are expressed mainly in the hypothalamus and mamillary bodies and their effect is excitatory. The activation of H1 receptors decreases membrane permeability for potassium and thus causes depolarisation. Most antihistamines produce sleepiness by their antagonistic effect on H1 receptors.
2) H2 receptors
These are metabotropic receptors couple to a Gs protein, they are expressed in neurons in many cortical areas, also in glial cells and the endothelium of cerebral capillaries.
3) H3 receptors
Found on presynaptic terminals they act as autoreceptors and limit further release of histamine. They are also present as heteroreceptors on noradrenergic, dopaminergic and cholinergic neurons, where they inhibit the release of the respective neurotransmitter.
_
Purinergic neurotransmitters
Some purines function as neurotransmitters – ATP, AMP or adenosine.
Purinergic receptors are present in most cells of our body. They regulate a plethora of functions – induction of proliferation, migration of neural crest cells, initiation of apoptosis or cytokine secretion.
We recognise three classes of purinergic receptors:
1) P1 receptors
2) P2X receptors
3) P2Y receptors
1) and 3) P1 receptors and P2Y receptors
These are G-protein coupled receptors. They are found mainly in the heart, kidneys, adipose tissue and brain. They differ in their main agonist, which is adenosine for P1 and ATp for P2Y. P2Y plays a role in the pathogenesis of chronic pain.
2) P2X receptor
This receptor is coupled to a non-selective cation channel and its main agonist is ATP. It is needed for maintaining postsynaptic excitatory responses. It is expressed in both neurons and glia and also in blood vessels of the CNS. This distribution could help coordinate nervous and vascular function in development and during disease.
It also plays a role in neuropathic pain.
_
Peptides
Peptide neurotransmitters are synthesised on ribosome in the neuronal body and are then transported via axonal transport to the synaptic terminal.
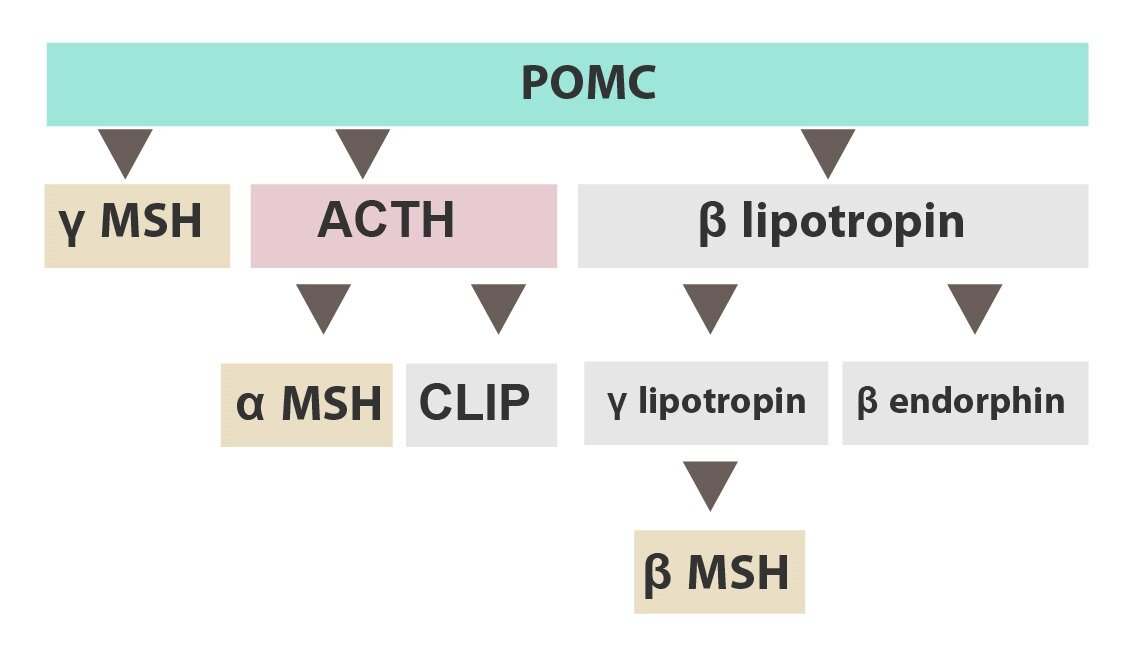
It is often the case that a large precursor molecule is synthesised and then spliced into smaller peptides. One such example is pro-opiomelanocortin (POMC), which is spliced into several neuropeptides such as
1) β-endorphin
2) MSH (melanocyte stimulating hormone)
3) ACTH (adrenocorticotropic hormone)
The group of peptide neurotransmitters is large, here are some other examples:
1) Opioid peptides: enkephalins, endorphins, dynorphin
2) Substance P
3) Neuropeptide Y
4) Somatostatin
5) Cholecystokinine
_
Endocannabinoids
Their main representative is anandamide (arachidonoylethanolamide, AEA) – amide of ethanolamine and arachidonic acid. The synthesis and degradation is complex and can be found in the literature.
Anandamide acts in the CNS and immune system via cannabinoid receptors:
1) CB1 receptors
Present mostly in the CNS presynaptically on GABAergic neurons they are coupled to a G-protein. Their activation inhibits the release of GABA into the synapse. This phenomenon is called depolarisation-induced inhibition suppression.
CB1 receptor agonists have orexigenic effects and stimulate the reward system.
2) CB2 receptors
Mostly present in the peripheral nervous system, hematopoietic and immune cells. They play a role in pain relief and immune response.
Historic note:
The structure of anandamide was described in 1992 by the Czech chemist Lumír Ondřej Hanus and the American pharmacologist William Anthony Devane at Hebrew University in Jerusalem. Its name is derived from the Sanskrit word ananda meaning bliss.
Subchapter Authors: Patrik Maďa and Josef Fontana