Content:
1. Introduction to the acid-base balance
2. Systems responsible for the maintenance of the acid-base balance
3. Laboratory tests of the acid-base balance status
4. Basic disorders of the acid-base balance and means of compensation
_
Introduction to the acid-base balance
Maintenance of the internal environment is one of the vital functions (it has same importance as circulation or respiration). In Subchapter 7/6 is pointed out that maintenance of stable pH, also called isohydria, is one of the basic components of the internal environment: (1) isohydria, (2) isovolumia (stable volume), (3) isoosmolarity (stable tonicity), and (4) isoionia (stable ion composition).
Proton concentration and pH
Maintaining of stable anion and cation concentrations in blood plasma is denoted as isoionia. Maintaining of constant proton (H+) concentration is isohydria. pH is used for express concentration of the protons:
pH = – log c(H+)
Plasma and extracellular space concentrations of the protons are held in very narrow physiologic range. There is 40 nmol/l of protons in the arterial blood physiologically (note that concentrations of other plasma ions, e.g. [Na+] = 140 mmol/l or [HCO3–] = 25 mmol/l, are three orders of magnitude higher). pH could be easily calculated as follows:
pH = -log 40 x 10-9 mol/l
pH = 7,4
Physiologic range of the pH is 7,36-7,44.
Value of pH higher than 7,44 in arteries is denoted as alkalemia, pH lower than 7,36 is acidemia. Extensive deviations of pH value can cause serious consequences. For example change of protein structure (i.e. enzymes), membranes permeability, and electrolyte distribution. Value of pH in arterial blood higher than 7,8, resp. lower than 6,8 are incompatible with life.
Values mentioned above apply for arterial blood. Values differ in different body compartments hence there are different H+ concentrations. There is quite variable and lower pH value intracellular, it is about 7,0 ([H+] = 100 nmol/l). Intracellular pH compared to arterial pH gives difference 0,4. This corresponds to fact that there is 2,5 fold difference between intracellular and arterial H+ concentration. This concentration gradient drives the movement of H+ from cells to blood. Therefore it is not surprising that venous pH and pH of interstitial fluid is lower (i.e. more acidic) than arterial pH. Approximate value is 7,35.
Acids and bases in the body
Acid is defined as molecule that can cleave off H+ (Arrhenius) or donor of H+ (Brönsted). Base is au contraire molecule that can cleave off OH– (Arrhenius) or acceptor of H+ (Brönsted).
Source of acids in the body is chiefly metabolism, source of bases is predominantly nutrient.
Acids and bases undergo either (1) metabolic conversion (e.g. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or (2) excretion from body.
Three types of reactions can be distinguished from point of view of the acid-base balance. (1) proton-productive, (2) proton-consumptive, (3) proton-neutral. Examples follow:
1) Proton-productive reactions
a) Anaerobic glycolysis in muscles and erythrocytes
Glucose → 2 CH3CHOHCOO– + 2 H+
b) Ketogenesis – production of ketone bodies
Fatty acids → ketone bodies + n H+
c) Lipolysis
TAG → 3 FA + glycerol + 3 H+
d) Ureagenesis
CO2 + 2 NH+4 → urea + H2O + 2 H+
2) Proton-consumptive reactions
a) Gluconeogenesis
2 lactate + 2 H+ → Glc
b) Neutral and dicarboxylic amino acids oxidation
3) Proton-neutral reactions
a) Complete glucose oxidation
b) Lipogenesis from glucose
Human organism (healthy or not) every day produces great quantities of acids – source of protons. Organism is acidified by these processes:
1) Complete oxidation
Carbon skeleton → CO2 + H2O → HCO3– + H+
2) Incomplete oxidation
Carbohydrates → glucose → pyruvate, lactate + H+
Triacylglycerol → fatty acids, ketone bodies + H+
Phospholipids → phosphate + H+
Proteins → amino acids→ sulphate, urea + H+
Acids can be divided into two groups: (1) volatile acids (respiratory acids), (2) non-volatile acids (metabolic acids).
The most important volatile acid is carbonic acid (H2CO3). H2CO3 is produced by reaction of carbon dioxide (CO2 is acid-forming oxide) with water. 15 000 – 20 000 mmol CO2 (therefore same amount of carbonic acid) is produced every day. Respiratory system however very efficiently eliminates it. This justifies the term volatile acid.
Two groups are distinguished among non-volatile acid: (1) organic, and (2) inorganic. 1 mmol/kg of body weight is produced every day. Non-volatile acid could be either (1) metabolised, or (2) excreted (using mainly kidneys).
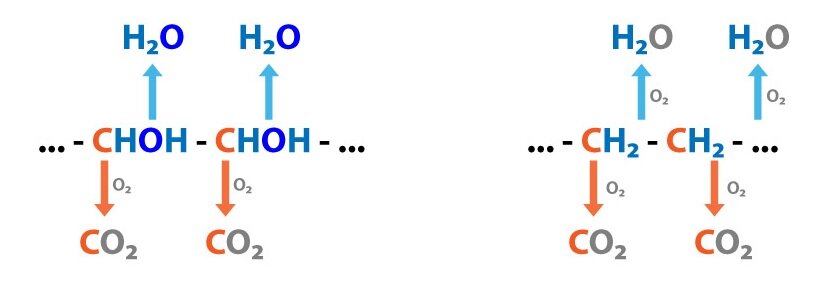
Organic non-volatile acids are for example: (1) lactic acid, (2) fatty acids, (3) ketone bodies (acetoacetic acid, β-hydroxybutyric acid). They are continually produced by metabolism (incomplete oxidation of TAG, carbohydrates, proteins). As organic non-volatile acids are products of metabolism in normal conditions they are oxidized completely to CO2 and H2O. Therefore they have no influence on proton overall balance.
Inorganic non-volatile acids are: (1) H2SO4 (sulphuric acid is produced by oxidation of sulfhydryl groups – e.g. in amino acids that contain sulphur, i.e. cysteine, methionine), (2) H3PO4 (phosphoric acid is produced by hydrolysis of phosphoproteins, phospholipids, nucleic acids). Inorganic non-volatile acids are predominantly excreted in urine.
You should notice now that ATP production is coupled with H+ production. Human body is evolutionary capable to handle acid load.
_
Systems responsible for maintenance of the acid-base balance
Several systems maintain constant pH. The list below is made according to order when they act:
1) Chemical buffering systems
Buffers react immediately – acute regulation. Capacity of buffers is not indefinite that is why chemical buffers act only in the short-term. Chemical buffering systems deal with pH deviations in common metabolism.
2) Respiratory system
Respiration reacts in 1-3 minutes. Respiratory system regulates carbon dioxide. Respiration is able to change pCO2 by its elimination or retention. Respiratory centre is in brainstem.
3) Kidneys
Kidneys react in hours-days. Their role in acid-base balance is very complex.
4) Liver
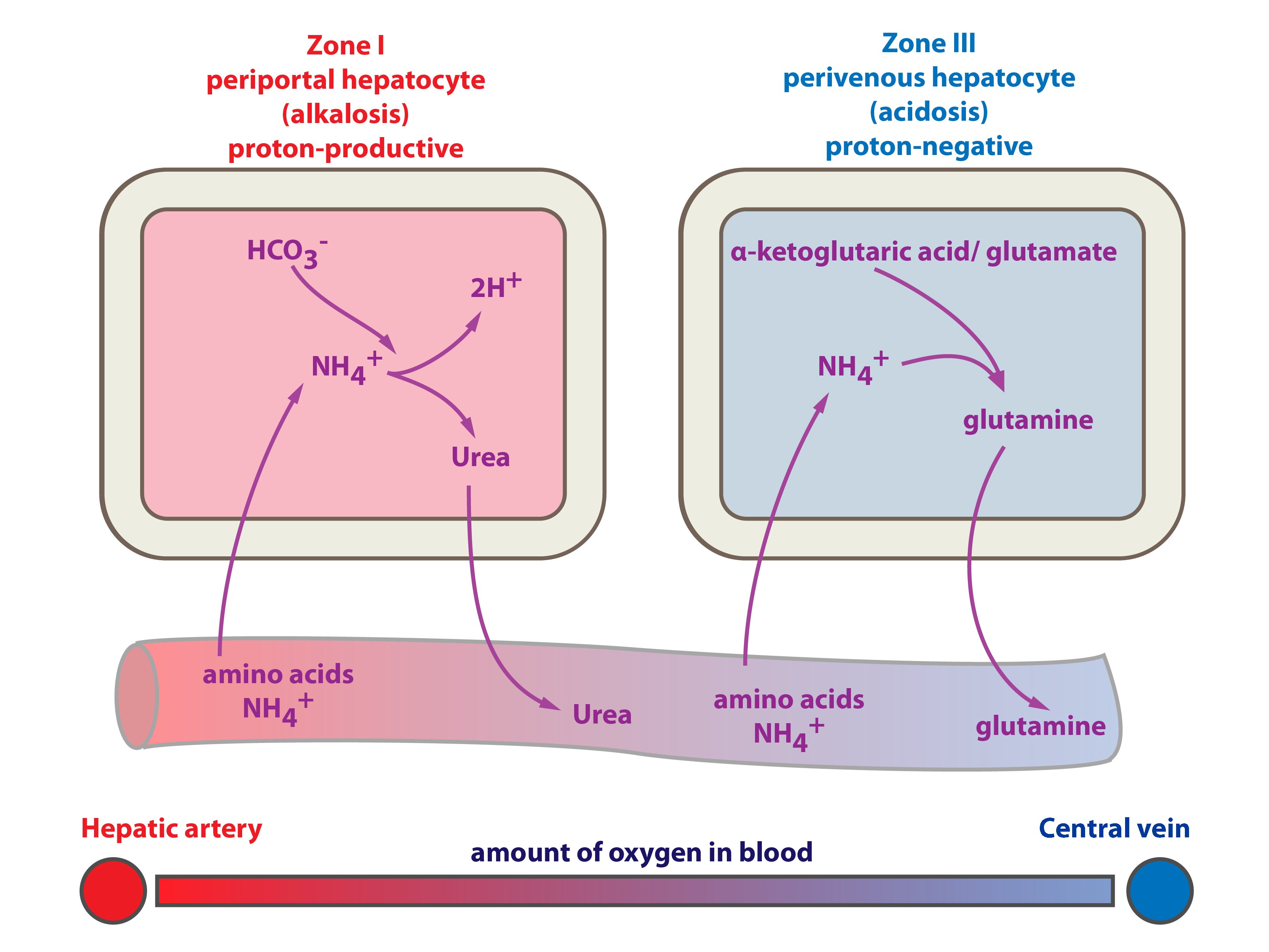
Liver is pivotal organ of the energetic metabolism it also have important influence on the acid-base balance. Liver is the most important tissue where ammonium is detoxified in both (1) urea cycle, and (2) glutamine synthesis. Which one of these fates of ammonium is favoured closely depends on status of the acid-base balance:
a) NH4+ → urea + 2 H+ → acidification of the body
CO2 + 2 NH4+ → CO(NH2)2 + 2 H+ + H2O
H+ + HCO3– → H2O + CO2 (consumption of bicarbonate–)
b) NH4+ → glutamine synthesis → H+ is not produced, glutamine is taken up by the kidneys. In the kidney is H+ excreted as NH4+
5) Myocardium
Myocardium influences acid-base balance through lactate and ketone bodies oxidation.
Buffering systems
Buffers are substances capable of releasing and binding H+. Short-term and acute changes in acid-base balance can be balanced by buffers. Each buffer keeps its particular pH. This pH could be calculated by means of the Henderson-Hasselbalch equation:
pH = pK + log [conjugated base]/[acid]
Henderson-Hasselbalch equation for bicarbonate buffer (HCO3–/CO2):
pH = pKH2CO3 + log ([HCO3–] / [H2CO3])
pH = pKH2CO3 + log ([HCO3–] / α x pCO2)
α is conversion factor, that is used for calculation of molar concentration (mmol/l) from partial pressure of CO2 (pCO2). α = 0,226 for pCO2 in kPA, α = 0,03 for pCO2 in mmHg).
pH = pK ± 1 is range where buffers work optimally.
In Henderson-Hasselbalch equation above you should notice that for pH that buffers keep depends primarily on ratio of conjugated base and acid (Of course concentration of each component is important but not that much). Therefore it is really important to know the ratio. Ratio of conjugated base and acid could be calculated from relation between pH and pK. For example bicarbonate buffer (pH = 7,4; pK = 6,1):
pH = pKH2CO3 + log ([HCO3–] / [H2CO3])
7,4 = 6,1 + log ([HCO3–] / [H2CO3])
1,3 = log ([HCO3–] / [H2CO3])
[HCO3–] / CO2 ≈ 20 / 1
The ratio in bicarbonate buffer is 20:1 (HCO3– : CO2)
There are several buffer systems in the body. The most important include: (1) bicarbonate buffer (HCO3–/CO2), (2) haemoglobin buffer (in erythrocytes), (3) phosphate buffer, (4) proteins, and (5) ammonium buffer. Their importance differs as it depends on localization.
Main buffer systems according to body compartments.
|
Localization |
Buffer |
Commentary |
|
ISF |
Bicarbonate | Buffers metabolic acids |
| Phosphate | Low concentration – limited significance | |
| Proteins | Low concentration – limited significance | |
|
Blood |
Bicarbonate | Buffers metabolic acids |
| Haemoglobin | Buffers CO2 (carbonic acid production) | |
| Plasma proteins | Minor | |
| Phosphate | Low concentration – limited significance | |
|
ICF |
Proteins | Significant buffer |
| Phosphate | Significant buffer | |
|
Urine |
Phosphate | Responsible for majority of the titratable urine acidity |
| Ammonium | Significant: elimination of ammonium nitrogen and protons; cation |
_
Following table shows buffering capacity of blood buffers.
Blood buffers and their buffer capacity
|
Buffer |
Plasma |
Erythrocytes |
Together |
| HCO3– / CO2 |
35 % |
18 % |
53 % |
| Hb / Hb-H+ |
– |
35 % |
35 % |
| Plasma proteins |
7 % |
– |
7 % |
| Inorganic phosphate |
1 % |
1 % |
2 % |
| Organic phosphate |
– |
3 % |
3 % |
|
43 % |
57 % |
100 % |
_
Because of fact that all buffer systems are in equilibrium any kind of drift in pH causes response in all buffer systems. Any concentration change of any component of any buffer influences both pH, and all buffer systems.
Bicarbonate buffer (HCO3–/CO2)
Bicarbonate buffer is the most important buffer system in blood plasma (generally in the extracellular fluid). This buffer consists of weak acid H2CO3 (pK1 = 6,1) and conjugated base HCO3– (bicarbonate).
Bicarbonate concentration is given in mmol/l (average value is 24 mmol/l). Since carbonic acid is very unstable molecule measurement of its concentration is very difficult. H2CO3 is produced from CO2 hence it is possible to express carbonic acid concentration as partial pressure of CO2 (pCO2) because pCO2 is directly proportional to CO2 concentration. pCO2 is easily measured (kPa, mmHg). Average value in arterial blood is 5,3 kPa = 40 mmHg. pCO2 multiplied by α gives us molar concentration of dissolved CO2 (α = 0,226 for pCO2 in kPa, α = 0,03 if pCO2 for mmHg). Conversion relationship between mmHg and kPa is: 1 Pa = 0,0075 mmHg (i.e. 760 mmHg ≈ 100 kPa). In normal plasma pH is HCO3–/CO2 ratio 20 / 1.
Henderson-Hasselbalch equation for bicarbonate buffer:
pH = pK + log [conjugated base] / [acid]
pH = pK + log ([HCO3–] / [H2CO3])
pH = 6,1 + log ([HCO3–] / pCO2 x α)
pH = 6,1 + log (24 / 40 x 0,03)
pH = 6,1 + 1,3
pH = 7,4
HCO3–/CO2 is so called open buffer system. This means body is capable to actively alter both bicarbonate, and carbon dioxide. pCO2 is regulated by respiratory tract (by means of ventilation – respiratory rate and depth of breathing). HCO3– levels are altered by the kidneys and the liver. HCO3– could be both synthesized, and eliminated.
Now you should recall what is stated above: pH = pK ± 1 is range where buffers work optimally. This should mean that bicarbonate buffer would work best in range 5,1-7,1, but in pH 7,4 it is very effective because it is open That is: organism is able to actively change both components.
We use status of bicarbonate buffer for clinical evaluation of patient´s acid-base balance. (pH measurement, [HCO3–] a pCO2)
Protein buffers
Body proteins (plasma proteins and intracellular) are the most abundant and the most powerful buffer system in whole organism. Some amino acids have acid or basic side chains (His, Lys, Arg, Glu, Asp). Among blood proteins haemoglobin is the most important. It provides 35 % of buffering capacity of blood, remaining proteins provide only 7 %.
Role of erythrocytes and haemoglobin in the acid-base balance
Intensive change of blood gases occurs in working tissue. CO2 diffuses to erythrocytes. In the red blood cell CO2 either (1) binds to haemoglobin (and carbaminohemoglobin is formed), or (2) reacts with water. This reaction is catalysed by carbonic anhydrase (CA, carbonate dehydratase):
CO2 + H2O ↔ H2CO3
Produced carbonic acid dissociates:
H2CO3 ↔ HCO3– + H+
More than 70% of produced HCO3– leave erythrocyte using special HCO3–/Cl– antiport. That is bicarbonate is exchanged for Cl–. This process is called Hamburger´s effect (chloride shift). In carbonic acid dissociation H+ is produced. Generated protons are buffered by haemoglobin. Deoxygenated haemoglobin is stronger base than oxygenated thus deoxygenated is more capable of taking up protons.
In lungs HCO3– is changed to CO2, using enzyme CA. CO2 is exhaled. Reaction HCO3– → CO2 + H2O demands H+. Protons for this process are taken from haemoglobin which affinity to H+ has lowered just when it arrived to lungs where is high pO2 and haemoglobin become oxygenated. Reaction catalysed by carboanhydrase has reverse course in lungs in comparison to other tissues:
HCO3– + H+ → CO2 + H2O
More information are in Chapter 6.
Phosphate buffer
Phosphate buffer consists of inorganic and organic bound phosphate (i.e. esters of organic substances, e.g. AMP, ADP, and ATP). Phosphate buffer is important intracellular and urine buffer. In blood it accounts for only 5 % of buffering capacity.
Urine buffers
There are two important urine buffers: (1) ammonium buffer (NH3/NH4+) and (2) phosphate buffer. Every day is excreted 30-50 mmol of NH4+. This is important because excretion of NH4+ is significantly regulated when the acid-base balance is disturbed. That is excretion of ammonium could be much decreased or much increased. In acidosis is glutaminase activated in the kidneys. Glutaminase splits glutamine to glutamate and NH3. NH3 is then eliminated to the urine. This process includes also the liver, where less urea and more glutamine is produced in acidosis. Every day is excreted 20 mmol of phosphates (i.e. titratable urine acidity). Physiologic urine pH is 4,4-8,0.
Role of the respiratory tract in maintaining the acid-base balance
Every day is exhaled approximately 15-20 moles of CO2 by the respiratory system. CO2 is well soluble in water therefore its concentration in both alveoli and arterial blood is the same (i.e. pCO2 = 5,33 kPa = 40 mmHg). In venous blood is pCO2 6,13 kPa = 46 mmHg.
pCO2 depends – besides other things – on the pulmonary ventilation (= respiratory minute volume). Pulmonary ventilation is defined as respiratory rate (RR) multiplied by tidal volume (VT). For understanding following concept you should recall that pH of buffer depends on ratio of its components (e.g. HCO3– : pCO2) and so when ratio changes, pH changes consequently. You can now easily deduce that:
1) increased ventilation leads to drop in pCO2 and that leads to alkalisation (increased pH)
2) decreased ventilation leads to accumulation of CO2 → increased pCO2 and that leads to acidification (decreased pH)
There are many ways for controlling breathing. One of them is chemical control. Chemoreceptors check both pCO2, and pO2. Increased pCO2 activates breathing centre. Sensitivity of chemoreceptors is decreasing when pCO2 is 8 kPa or higher. Only remaining stimulus for breathing centre is decreased pO2.
Role of the kidneys in maintaining the acid-base balance
Chemical buffers are capable of stopping increase in acids or bases. Buffers however are not capable of eliminating those acids and bases from body. Respiratory tract can eliminate (or cumulate) volatile carbonic acid by means of eliminating CO2 (or cumulate it). Only the kidneys are able to clean the body from non-volatile (metabolic) acids (i.e. phosphoric acid, sulphuric acid, uric acid, …). Thus preventing acidosis. In addition the kidneys are only organ that is efficiently capable of solving alkalosis (respiratory system btw offers another option, i.e. stop breathing).
The kidneys take part in maintaining the acid-base balance by means of:
1) Reabsorbing, excreting and producing bicarbonate
2) Excreting or producing H+
You should notice that loss of bicarbonate is the same as acquire of H+ and production of bicarbonate is the same as loss of H+. It is shown below that these processes are connected (e.g. excretion of H+ in proximal tubule is connected with reabsorption of HCO3– in the same place or excretion of H+ in distal tubule is connected with production of HCO3– in the same place). Next important concept is that higher bicarbonate concentration increases pH, lower bicarbonate concentration decreases pH.
In this section are in detail described basic processes as reabsorption of bicarbonate, new bicarbonate production, ammonium ion production, proton excretion in kidneys, bicarbonate secretion.
Bicarbonate reabsorption
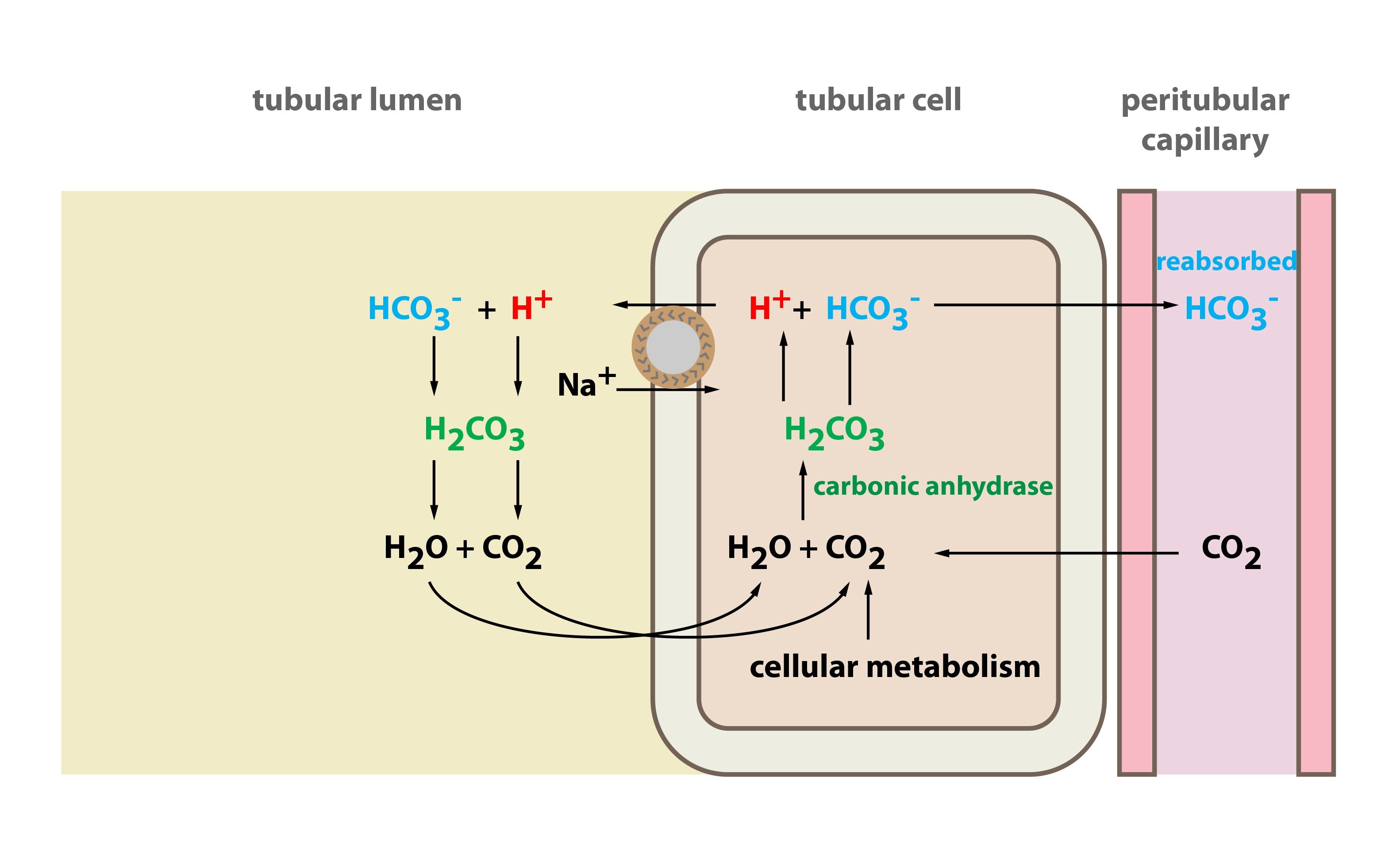
Bicarbonate reabsorption takes place in proximal tubule cells. In glomerular ultrafiltrate there is filtered bicarbonate. To the lumen of the proximal tubule is transported H+. H+ is transported by Na+/H+ antiport H+ reacts with HCO3– and H2CO3 is thus produced. H2CO3 split up into H2O and CO2. Water and carbon dioxide get through apical membrane of tubular cells. Inside these cells H2CO3 is again produced. H2CO3 dissociates into HCO3– and H+. Now their fates get different: (1) H+ becomes again substrate for Na+/H+ antiport and it is transported again to the lumen of the proximal tubule where it can “catch” another bicarbonate molecule. (2) Bicarbonate however traverse basolateral membrane into interstitial fluid (and then to the blood of the peritubular capillaries). Bicarbonate gets through basolateral membrane using either Na+/3 HCO3– cotransport, or anion exchanger (Cl–/HCO3– exchange).
Together it can be stated: for one secreted H+, one Na+ and one HCO3– are resorbed. Na+ is transported to the blood among other things by active transport – i.e. Na+/K+ ATPase.
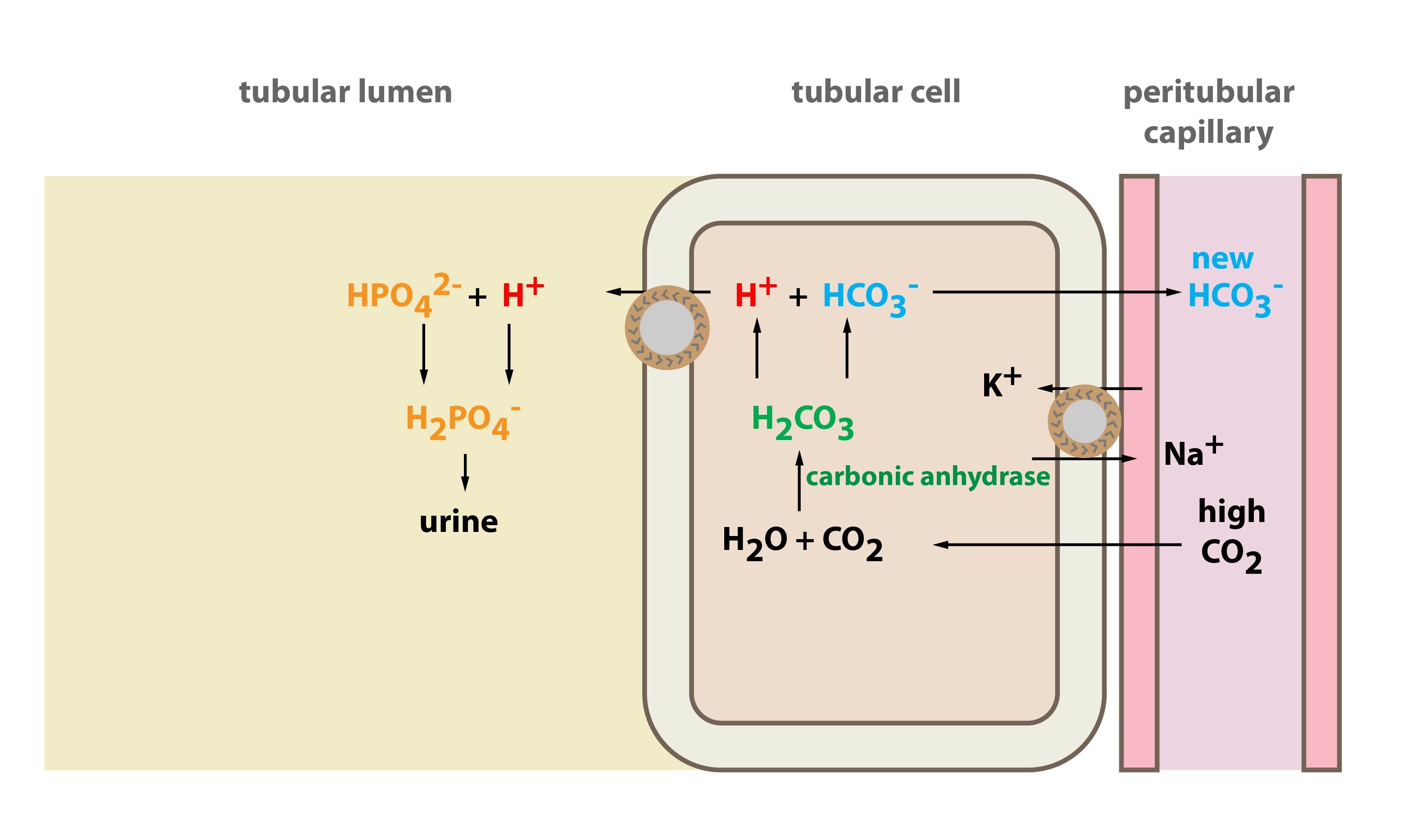
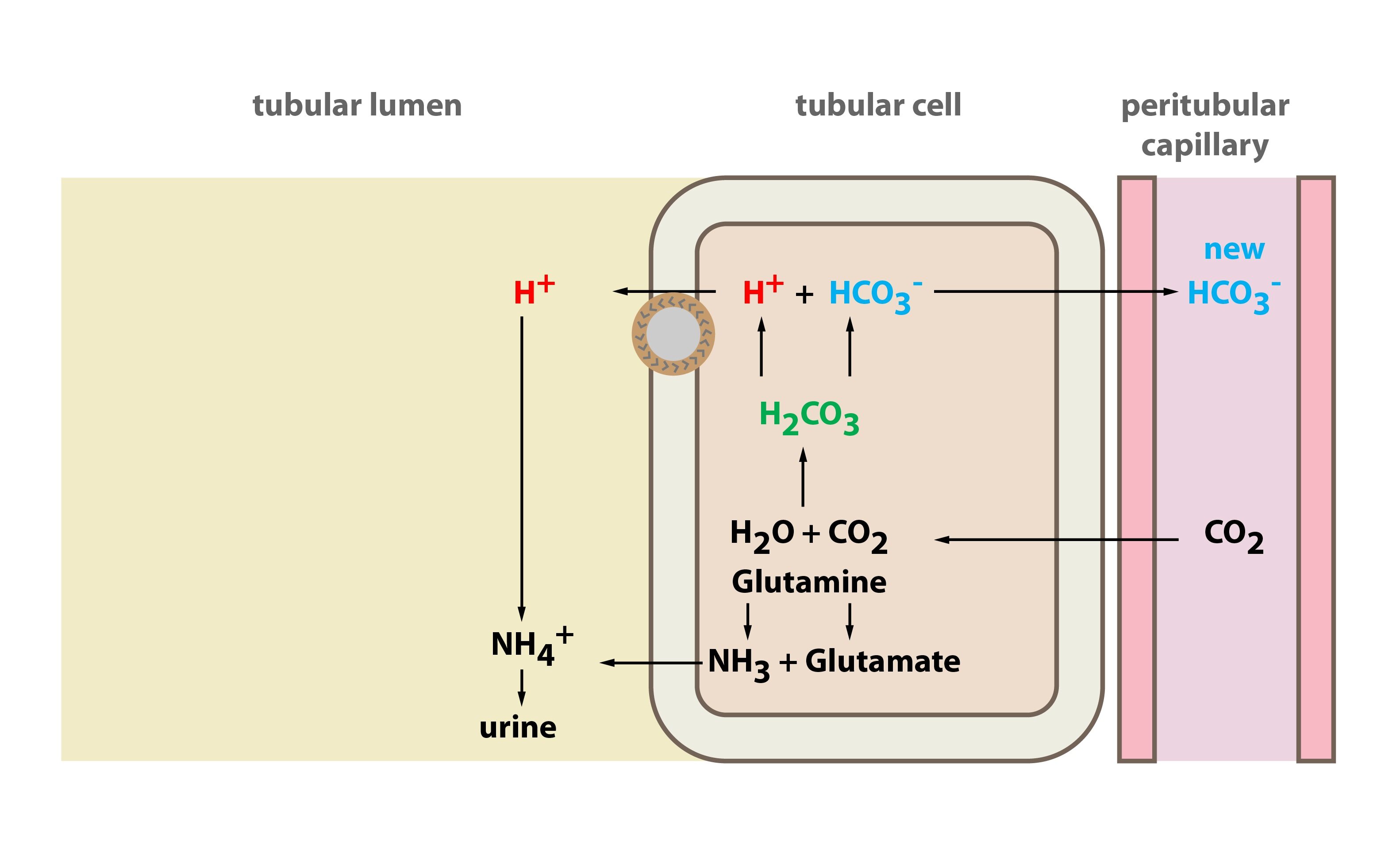
New bicarbonate production (connected with H+ excretion)
New bicarbonate production takes place in intercalated cells type A of distal tubule and collecting duct. These cells absorb CO2 from the blood and inside the cells carbon dioxide reacts with water and carbonic acid is thus produced, catalysed by the enzyme carboanhydrase. Carbonic acid dissociates to H+ and HCO3–. H+ has totally different fate than bicarbonate: (1) H+ is excreted by the H-ATPase to the urine. This process is active, hence it consumes ATP. In order to eliminate as much H+ as possible it is necessary to buffer H+ in the urine. The most important buffers in the urine are ammonium and phosphate buffer. (2) Produced bicarbonate is transported to the blood in peritubular capillaries exchanged for Cl– (Cl–/HCO3– exchanger in basolateral membrane). Aldosterone stimulates H+ secretion (and therefore H+ excretion).
Ammonium ion excretion
This process uses ammonium generated in glutamine metabolism in tubular cells. For every metabolised glutamine two ammonium ions and two bicarbonates are produced. Bicarbonates are transported to the blood, whilst ammonium ions are excreted to the blood.
Proton excretion in the kidneys
Both bicarbonate resorption, and new bicarbonate production (both mentioned above) need transport of H+ (protons) to the tubules (protons are derived from carbonic acid dissociation). Precise mechanism is however quite different.
In the cells of the proximal tubule the transport of proton to the lumen is based on its exchange for Na+. On the basolateral membrane act Na+/K+-ATPase and HCO3–/Cl– exchanger.
In the intercalated cells type A (in the distal tubule and the collecting duct) the transport of proton to the lumen is based on active transport (H+-ATPase). Aldosterone promotes (1) excretion of H+ and K+ in the distal tubule and the collecting duct and (2) reabsorption of the sodium (and water).
The result of both described processes is generation of high concentration gradient for H+, i.e. in the urine there is thousand times higher concentration of protons than in the cells/blood. This thousand fold gradient is however maximal, thus the lowest achievable pH of the urine is 4,4 (40 μmol/l H+) – compare this value with value of the pH in blood: 7,4 (40 nmol/l H+).
Bicarbonate secretion
In conditions of rising pH (alkalosis) type B of the intercalated cells start to act. They secrete bicarbonate and gain H+. These mechanisms are absolutely inverse than processes described in the type A of the intercalated cells (see above). Even in alkalosis nephrons however excrete less bicarbonate than they retain.
We can summarize that extracellular pH is kept by the buffer systems and involved organs. These systems maintain pH value 7,36-7,44. The respiratory system modulates pCO2 and the kidneys modulate concentration of bicarbonate.
_
Laboratory assessment of the acid-base balance status
Laboratory assessment of the acid-base balance status consists of: (1) acid-base balance parameters (pH, [HCO3–], pCO2, pO2 a BE) and (2) examination of other substances that can alter acid-base balance. These substances are for example:
1) Cations: [Na+], [K+], [Ca2+], [Mg2+]
2) Anions: [Cl–], [lactate], albumin
3) Metabolites: [urea], [creatinine], [ketone bodies]
Acid-base balance status is assessed according to the status of the bicarbonate buffer. It is so called examination of the ABR parameters by Astrup (ASTRUP).
This examination is used for assessment of the actual status of the acid-base balance in particular patient. The specimens are measured in analysers and these particular specimens are called “Astrup” after one of the first acid-base balance theory authors. Some parameters are not measured directly but calculated by software using Henderson-Hasselbalch equation.
The specimens are obtained from arterial blood (a. radialis or a. femoralis), sometimes it is necessary to collect capillary blood too. We can analyse only non-clotting blood (for this purpose heparin is added). Arterial blood must not contain air bubbles (because presence of air could alter pO2 (increase), pCO2 (decrease) and pH (increase)) and analysis should take place as soon as possible.
Normal arterial Astrup results:
Directly measured values:
1) pH = 7,36 – 7,44
2) pCO2 = 4,8 – 5,9 kPa (35-45 mmHg), average is 5,3 kPa (40 mmHg)
pCO2 < 4,8 kPa is denoted as hypocapnia
pCO2 > 5,9 kPa is denoted as hypercapnia
3) pO2 = 9,9 – 13,3 kPa (80-100 mmHg)
Calculated values:
4) [HCO3–] = 22-26 mmol/l
5) BE = 0 ± 2,5 mmol/l
BE (base excess)
Base excess is defined as number of moles of strong acid that is needed to add to one litre of fully oxygenated blood to achieve pH 7,4 when pCO2 is 5,3 kPa and temperature is 37°C. BE is optimal quantity for assessing metabolic component of acid-base balance. Normal values are 0 ± 2,5 mmol/l. Negative value indicates excess of acids (so the value is negative). Excess of acids is metabolic acidosis. Positive value indicates excess of bases (base excess), hence metabolic alkalosis.
There is however one very similar quantity – base deficit (BD). It indicates deficit of bases in mmol/l.
Ions and pH
Ion composition of extracellular fluid is closely related to the acid-base parameters. Kalemia is influenced most by acid-base balance disturbances.
Acidosis leads to efflux of K+ from the cells. That leads to the hyperkalemia. K+ is lost in the urine. When acidosis is treated quickly alkalization of the body leads to the influx of K+ back to the cells. That leads to hypokalemia. Hypokalemia is mostly dangerous for the heart – membrane signal transmission.
Alkalosis leads to efflux of H+ from the cells. To maintain electrical charges the same, K+ enter the cells in order to replace H+. Thus alkalosis leads to the hypokalemia. K+ is excreted in the urine instead of H+.
Anion gap (AG)
Anion gap is a quantity which is almost equal to the sum of concentrations of “unmeasurable” anions (albumin – plasma proteins, phosphates, sulphates, organic anions). Unmeasurable is not accurate term, more precise is commonly non-measured.
AG is calculated as follows:
AG = ([Na+] + [K+]) – ([Cl–] + [HCO3–])
Na+ (140) + K+ (5) = Cl– (105) + HCO3– (25) + AG (15)
Normal AG: 14 ± 2 mmol/l
Anion gap is used for assessing causes of the metabolic acidosis. One of the causes is the accumulation of the acids. Concentrations of some of them are not commonly measured. When there is accumulation of commonly non-measured acids unexpected rise in difference of measured cations and anions. This increase in difference could be revealed by AG. Therefore when there is increased AG it indicates that commonly non-measured acids accumulated. They become part of AG. Thus greater AG indicates acidosis.
Increased AG is caused by:
1) Increase in concentration of ions that physiologically make the AG
2) Presence of new anions
This method is unfortunately dependent on accuracy of the measurements. Little mistake in big numbers lead to greater mistake in the result. There are particular situations when we need to measure commonly non-measured acids (anions) concentrations. Then we measure:
1) Lactate in tissue hypoxia
2) 3-hydroxybutyrate in diabetic ketoacidosis
3) Phosphates and sulphates in renal failure
_
Basic disturbances in the acid-base balance and compensation
Acidosis is process that leads to the drop in pH value. Alkalosis is au contraire process that leads to the increase in pH value. Acid-base balance parameters are calculated for plasma which pH is alkalic, i.e. pH = 7,4 (H+ concentration is 40 nmol/l). Thus you should notice that even alkalic pH (e.g. 7,2) is acidosis!
Respiratory disturbances are indicated by shifts in pCO2 (respiratory disorder – hyper- or hypocapnia). Metabolic disturbances are indicated by shifts in BE (or [HCO3–])
Four basic acid-base balance disturbances are distinguished:
1) Respiratory acidosis (RAC): decreased blood pH; its primary cause is increased pCO2
2) Respiratory alkalosis (RAL): increased blood pH; its primary cause is decreased pCO2
3) Metabolic acidosis (MAC): decreased blood pH; its primary cause is decreased BE ([HCO3–])
4) Metabolic alkalosis (MAL): increased blood pH; its primary cause is increased BE ([HCO3–])
Compensation and correction of acid-base disturbances
Compensation is process when organism tries to maintain almost normal pH. Compensation is performed by system that works normally, i.e. the acid-base disturbance is caused by the other system. Compensation thus means metabolic disturbances are compensated by respiratory system and respiratory disturbances are compensated by metabolic components of acid-base balance.
Correction is solving the acid-base problem in the spot where it started. I.e. metabolic disturbances are solved by metabolic component of acid-base balance. In the body correction takes place only in metabolic disorders, i.e. metabolic disorder is corrected by another component of the metabolic component of acid-base balance.
Doctors however are capable of correction of both respiratory, and metabolic disturbances. Respiratory disturbances can be solved by artificial ventilation, metabolic disturbances by for example dialysis.
When both correction, and compensation are performed by the body itself, pH never normalizes completely..
Respiratory acid-base balance disturbances
All the people (healthy or not) produce every day large quantities of acids. The most important acid is CO2. Carbon dioxide is normally eliminated from the body by the respiratory system. When respiratory system is not capable of normal CO2 elimination (carbon dioxide could be eliminated too much or too few) respiratory acid-base balance disturbances come into existence.
Normal pCO2 is 4,8-5,9 kPa (35-45 mmHg). pCO2 lower than 4,8 indicates respiratory alkalosis, pCO2 higher than 5,9 indicates respiratory acidosis.
Respiratory disturbances are compensated by the kidneys. The kidneys retain or excrete HCO3– in order to (1) keep ratio HCO3– : pCO2 and (2) draw pH nearer to the normal values. Renal compensation needs hours to days for full development.
Respiratory acidosis (RAC)
Respiratory acidosis emerges when the lungs eliminate too few CO2 (it usually occurs in hypoventilation). Low CO2 elimination leads to increased pCO2 in the blood (hypercapnia). Increased pCO2 causes decreased pH.
Causes of RAC are for example:
1) Loss of functional lung parenchyma (pneumonia, cystic fibrosis, emphysema)
2) Airway obstruction (loss of tonus of tongue muscles)
3) Insufficient ventilation (e.g. neuromuscular disorders, CNS disorders, intoxications (opiates), asthmatic paroxysm)
4) Thorax movement restriction (e.g. spine deformities)
Organism compensates RAC by increased HCO3– concentration in the blood by means of increased resorption and increased production in tubular cells of the kidneys (acidic urine is produced). Thus pH of the blood is drawn nearer to the normal values.
Causes of RAC mentioned above can sometimes cause decreased pO2 too. Tissue hypoxia leads to the metabolic acidosis caused by accumulation of lactate, thus it is called lactate acidosis (see below).
Respiratory alkalosis (RAL)
Respiratory alkalosis is caused by hyperventilation. Hyperventilation causes increased elimination of carbon dioxide and that leads to hypocapnia (decreased pCO2).
There is one important aspect concerning calcium. One of the important buffers in blood is albumin. You should recall that albumin binds approximately 50 % of plasma calcium. When pH changes, albumin binds or releases H+ and therefore calcemia is changed. This is very important in RAL. In this condition ratio between ionised and bound calcium is changed. In RAL is decreased ionised calcium hence hypocalcemia develops. Hypocalcemia could cause muscle spasms.
Causes of RAL are for example:
1) Hyperventilation due to psychic reasons (exhalation of the carbon dioxide = exhalation of the emotions) or hyperventilation due to the high altitude (i.e. breathing in lack of oxygen). In both principles pCO2 is lowered and you know that low pCO2 is alkalosis. Interestingly HCO3– is slightly lowered as well. This is because pCO2 is lowered and thus – to keep equilibrium – part of bicarbonate is converted to CO2. (HCO3– + H+ ↔ CO2 + H2O). Ions H+ needed for this reaction are provided from non-bicarbonate buffers.
2) CNS trauma
3) Salicylates poisoning (Aspirin) – fever, etc…
Compensation is decreased HCO3–. This is provided by larger excretion of HCO3– by the kidneys.
Metabolic acidosis (MAC)
Metabolic acidosis is the most common acid-base balance disorder. It is indicated by decreased pH (increased H+) and negative BE ([HCO3–]). BE is the best marker for assessing metabolic component of the acid-base balance. It can be stated that metabolic acidosis is pH that is too acidic compared with given pCO2 (i.e. metabolic component must be always assessed with knowledge of pCO2 in particular patient).
General causes of MAC:
1) Accumulation of “metabolic” acid. Anion of this acid eliminates bicarbonate.
2) Loss of bicarbonates (this loss of anion is accompanied by loss of cation, it is not surprising that most abundant cation (Na+) is lost mostly)
3) Loss of cations, predominantly Na+. This is compensated by decrease of bicarbonate
Every acid in the body apart from carbonic acid is so called metabolic acid. Metabolic acids are non-volatile, therefore they have to be neutralized and either metabolised, or eliminated by kidneys.
Bicarbonate are lost most commonly from the GIT. Duodenal and pancreatic juice have abundant bicarbonates. Normally high concentrations of bicarbonate in these juices neutralize low pH of chyme from stomach. Normally bicarbonates are resorbed in small intestine. There are however some diseases of the GIT (diarrhoea, short intestine syndrome, etc…) when bicarbonates are resorbed insufficiently. Bicarbonates can be lost in the kidneys too (renal tubular acidosis, adverse effect of diuretics – carbonic anhydrase inhibitors (acetazolamide)).
AG calculation is useful in differential diagnosis of MAC. Excessive production of acids leads to high AG. Elevated loss of bicarbonates has normal AG.
Now we mention some particular states that lead to MAC:
1) Hypoxia – lack of oxygen in tissues. This condition makes tissues to process glucose in anaerobic glycolysis. By-product of anaerobic glycolysis is lactate. Thus hypoxia leads to the lactate acidosis. Lactate acidosis is typical companion of RAC, shock or overdose of biguanides (metformin).
2) Excessive production of ketone bodies (acetoacetic acid and β-hydroxybutyric acid). This condition is caused by situations when glucose cannot be used as source of energy. This leads to excessive use of fatty acids as the main energy source. Thus excessive production of ketone bodies accompanies diabetes mellitus or starving. This condition is called ketoacidosis.
3) Alcohol intoxication (e.g. methanol, ethylene glycol). These alcohols are metabolised to strong organic acids (formic acid, oxalic acid). These acids release lots of H+. Oxalates can lead to renal failure. Overdose of salicylates (Aspirin) can cause MAC as well.
4) Renal insufficiency leads to condition when normally excreted acids are cumulated (sulphates, phosphates, some other anions). This is called renal acidosis.
5) Heavy diarrhoea
6) Loss of bicarbonates in the kidneys
In all these conditions at first buffering of excessive H+ takes place (it is carried out by bicarbonate and non-bicarbonate bases). Bicarbonate forms with H+ carbonic acid that forms CO2 and water, carbon dioxide is eliminated by the lungs.
Second step is compensation using hyperventilation. You should recall that hyperventilation leads to decreased pCO2 and decreased pCO2 means higher pH. This is often called Kussmaul acidotic breathing (breathing centre is stimulated by high H+ concentration).
Third step is correction by kidneys. Correction is launched in case that acidosis despite the compensation is still present. Kidneys perform (1) increased excretion of H+ and (2) new bicarbonate production (intercalated cells type A). This results in acidic urine.
Metabolic alkalosis (MAL)
Metabolic alkalosis is characterised by increased pH and risen BE. General causes are:
1) Loss of some anions (usually chlorides or proteins). This loss of anions is compensated by replenishing of other anions, predominantly bicarbonates (and increased bicarbonates mean alkalosis)
2) Increased cation concentration (most commonly Na+)
3) Increased alkali intake (e.g. alkalising medication – bicarbonate infusion)
Now we mention some particular states that lead to MAL:
1) Vomiting – loss of HCl (thus loss of H+). So called hypochloremic alkalosis develops (it is caused by diuretics as well (e.g. furosemide causes loss of K+ and Cl–)
2) Hypoproteinemia – proteins are anions thus decreased protein concentration is compensated by increased bicarbonate concentration (i.e. bicarbonates replenish missing anions). Hypoproteinemia is caused by liver failure, nephrotic syndrome or malnutrition.
3) Hyperaldosteronism. High aldosterone causes increased retention of Na+. This increased cation concentration must be accompanied by replenishing of anions because electroneutrality must be maintained (i.e. bicarbonate concentration is increased).
4) Iatrogenic bases delivery (e.g. HCO3– infusions)
At first buffering takes place. Compensation is second and body uses hypoventilation, thus less CO2 is exhaled and pCO2 rises, that leads to lowering pH. In case that alkalosis is not caused by kidneys, renal correction can take place. It is performed by higher excretion of bicarbonate (intercalated cells type B). One of serious consequences of alkalosis is hypokalemia that can lead to heart rhythm disturbances.
Mixed disturbances of acid-base balance
Mixed disturbances of acid-base balance are quite common. It is defined as either (1) combination of two or more basic disturbances of acid-base balance, or (2) combination of more causes that cause the same acid-base balance disturbance, (3) or both.
As an example we can use hypoventilation that leads not only to the respiratory acidosis because less CO2 is exhaled but also to the metabolic acidosis because less O2 is delivered to the tissues.
Subchapter Authors: Josef Fontana and Petra Lavríková