Content:
1. Introduction to metabolism of water and minerals
2. Body water and its distribution, osmolarity
3. Regulation of extracellular fluid volume and sodium metabolism
4. Metabolism of selected ions – a chloride anion, potassium and magnesium cations
5. Calcium and phosphate metabolism
_
Introduction to metabolism of water and minerals
The internal environment can be defined as the composition of the fluid that bathes the cells. Maintaining a constancy in the internal environment of the human body – its constant composition, is absolutely essential. This is one of the vital functions such as breathing or blood circulation.
The basic components of the internal environment include:
1) Constant volume
2) Constant tonicity and constant ionic composition
3) Constant pH
In this chapter we will further pursue the first two points. The issue of maintaining constant pH will be discussed in detail in subchapter about acid-base balance.
_
Body water and its distribution, osmolarity
Body fluids
All the water in the human body is summarized under the concept of total body water (TBW). It constitutes 55-60 % of body weight in adults. Women have a lower TBW than men because they have a higher proportion of body fat (the same rule applies to people who are overweight or obese). Young children and pregnant women have an increased proportion of TBW, but, with old age, the proportion of water in human body decreases (we can overstate and say, that as we age, our bodies gradually dry up).
Total body water is divided into two basic groups – intracellular and extracellular fluid.
Intracellular fluid (ICF) contributes 2/3 of adult TBW while extracellular fluid (ECF) contributes the remaining 1/3 TBW. In neonates the distribution is reversed – ICF is 1/3 TBW, ECF 2/3 TBW.
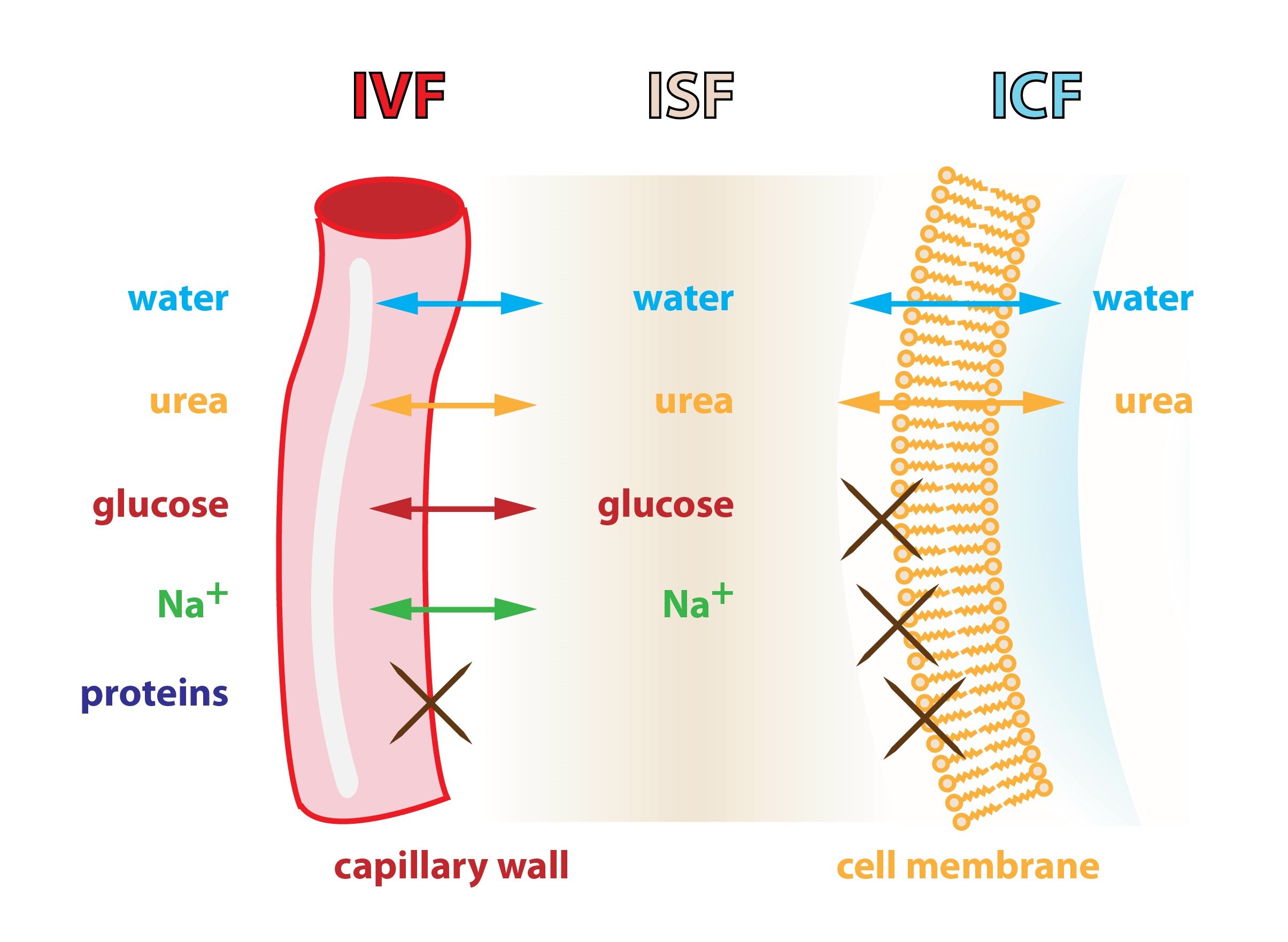
Extracellular fluid is further divided into the liquid stored in the arteries – intravascular fluid (IVF, plasma + lymph), contributing 1/4 ECF, and the interstitial fluid (tissue fluid) contributing 3/4 ECF.
Sometimes, the above is expressed as a percentage of body weight (% b. w.):
TBW (60 % t. h.) = ICF (40 % t. h.) + ECF (20 % t. h.)
ECF (20 % t. h.) = ISF (15 % t. h.) + IVF (5 % t. h.)
Certain fluids are held in a so called third space or preformed cavities. These include:
1) Cerebrospinal fluid
2) Liquid secreted to the gastrointestinal tract (digestive juices, …)
3) Synovial fluid
4) Fluid in the pleural, peritoneal and the pericardial cavity
Laws of water distribution and osmolarity
The water in the human body is moved by osmosis (most bodily barriers are semipermeable). The water content in the various compartments of the body is determined by the content of osmotically active particles – osmolarity. If deflection in osmolarity occurs anywhere in the body, an induced movement of water molecules through a semipermeable membrane occurs, in order to compensate for this deflection.
Figure captures three major compartments of the human body and the movement of individual substances across their borders.
Plasma osmolarity
Osmolarity is defined as the number of osmotically active particles in a liter – [mosm/l] = [mmol/l]. The physiological range is considered to be 280-295 mmol/l, with a most of the particles being low molecular weight substances – ions, nutrients and metabolites. Consequently we obtain an approximate idea of the osmolarity by a simple sum of concentrations of routinely measured substances -we obtain the so-called calculated osmolarity :
Calculated osmolarity = 2 [Na+] + [glucose] + [urea]
By cross-comparison of measured and calculated osmolarity values we obtain the so-called osmotic gap (OG):
Osmotic gap = measured osmolarity – calculated osmolarity
OG is comprised of all substances which we do not count when calculating the calculated osmolarity – its normal range is 4-12 mmol.
In many pathological conditions, we find OG increasing above normal physiological values. Two primary causes of this are:
1) Accumulation of foreign substances to the human body – For example, during a poisoning (e.g., ethanol, methanol, ethylene glycol)
2) Accumulation of substances, which are commonly found in the body, but their metabolism is altered – e.g., excessive catabolism in diabetes mellitus type 1 (overproduction of ketone bodies etc.).
Regulation of osmolarity
Fluctuation in body osmolarity is normally very low – only ± 1-2 %. The osmolarity of body fluids is regulated by the content of free water in the body. The main regulatory mechanisms are simple feedback via antidiuretic hormone (ADH, vasopressin) and thirst inducement.
Within lateral hypothalamus is an area we call the center of thirst. Neurons in this structure are able to monitor the osmolarity of the surrounding fluid. If surrounded by a hyperosmotic environment, shrinkage occurs in these cells (diffusion of water from the intracellular compartment into the extracellular hyperosmotic compartment), leading to a change in their activity, and ultimately inducing a strong feeling of thirst.
In the supraoptic area are similar neurons with osmoreception abilities. Those in hyperosmotic fluid increase its activity and through projections passing through the infundibulum into the neurohypophysis (posterior lobe of the pituitary gland) release antidiuretic hormone (ADH or vasopressin) from axons into adjacent capillaries. ADH subsequently flows via circulation to the kidneys, where it binds to its receptors on the cell membrane of cells in distal tubuli and collecting ducts of the kidneys. ADH causes increased permeability of the cells by water, which causes the so-called facultative water resorption and formation of more concentrated urine. These cells carry V2 membrane receptors for ADH, which get activated by ligand (ADH) binding that leads to activation of adenylate cyclase – cAMP formation – incorporation of aquaporins-2 (channels for water) to the apical membranes of cells. Aquaporins allow the passage of water by osmotic gradient from the lumen of the nephron into the hyperosmolar environment of the kidney medulla, where the water is drained by blood vessels. The result is an increase in urine osmolarity (the water is drawn from it, the solute is not) and a decrease in body osmolarity (for free water retention in the body). ADH simultaneously increases cell membrane permeability in cells of the collecting ducts for urea through increased expression transporters for urea (e.g. UT-A), which facilitate the reabsorption of urea into the interstitium medulla . This further enhances the absorption of water.
Antidiuretic hormone shows further effects in the human body, which we will not deal with at this point – they are discussed in other subchapters.
In addition to the above-described endocrine regulation an increase in body osmolarity affects a person’s behavior by inducing thirst → searching for and drinking fluids.
Disorders of antidiuretic hormone secretion
In case of an insufficient effect of ADH (insufficient secretion of hormone or absence of its receptors) appears excessive diuresis (polyuria – up to 30 liters per day) and excessive thirst (polydipsia). This disease is called diabetes insipidus.
The opposite condition is a syndrome of inappropriate secretion of ADH – SIADH (Syndrome of Inappropriate ADH secretion, Schwartz-Bartter syndrome), which leads to excessive secretion of ADH, which does not reflect the current state of osmolality. It leads to water retention, hypoosmolarity and dilutional hyponatremia (relative shortage of Na+ – usual amount is dissolved in large quantities of water). In more severe conditions brain damage develops due to the edema. This is due to intracranial pathologic processes or tumors producing ectopic ADH.
Clinical significance of plasma osmolarity
In clinical practice we frequently encounter osmolarity complications. For example, with each infusion, the doctor must take into account that the infusion has some osmolarity and that it will not harm the patient as a result. We always have to respect the tonicity of the infused solutions. By comparing the osmolarity of administered solutions with plasma osmolarity we can categorize the following solutions:
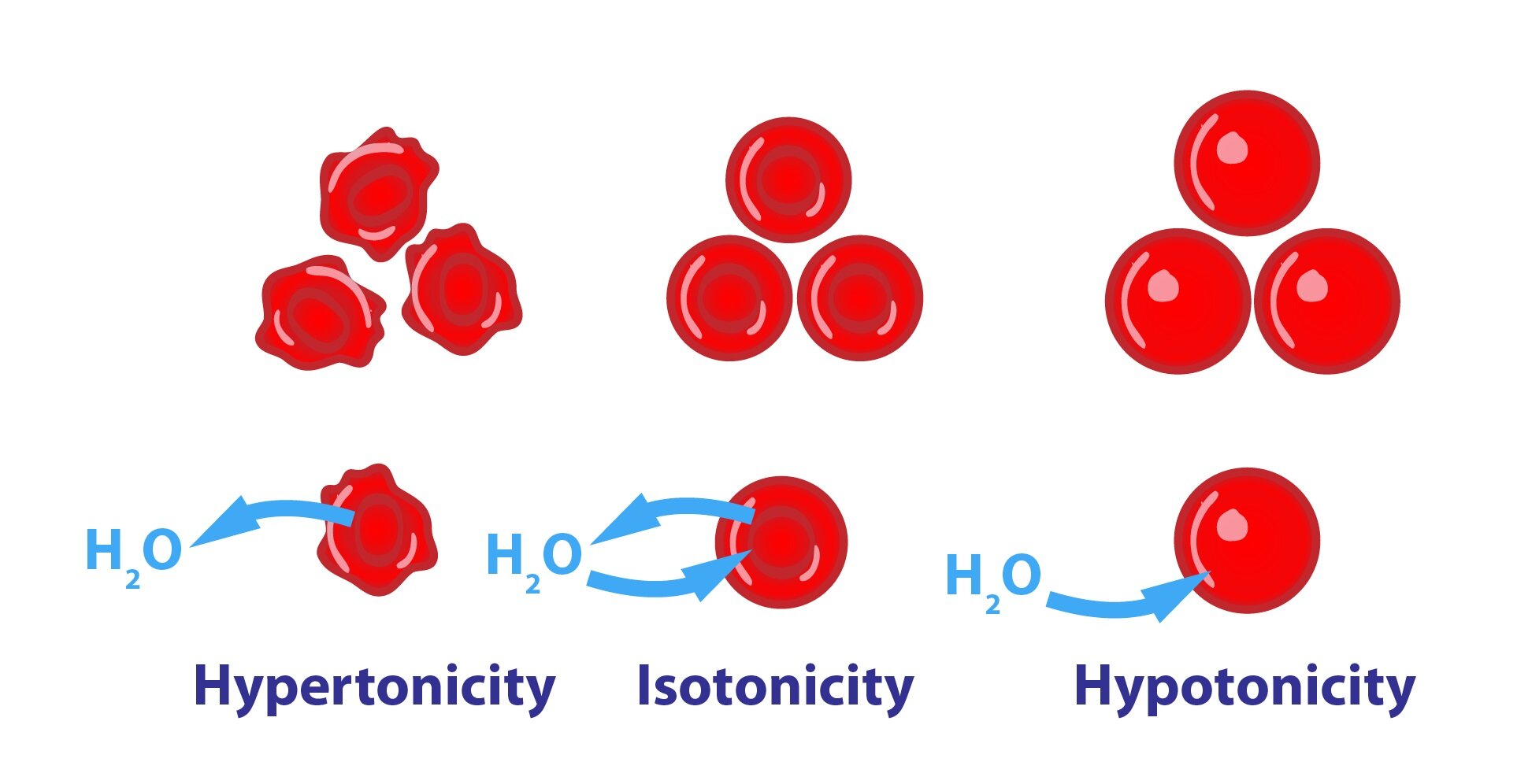
1) Isotonic solutions
Isotonic solutions have a similar osmolarity as plasma. These are the most frequently encountered in practice.:
a) Normal saline (NS) – 0,9% NaCl, 154 mmol/l Na+ and Cl–
b) 5% glucose – free water remains after glucose metabolism – de facto hypotonic solution
c) Ringer’s and Hartmann’s solution – ionic composition similar to plasma
2) Hypotonic solution
Excessive administration of hypotonic solution may cause hemolysis (destruction of red blood cells) and brain edema. Examples of hypotonic solutions: NS 1/2, R 2/3, H 2/3.
3) Hypertonic solutions
Hypertonic solutions can irritate the wall of blood vessels and cause damage to the CNS. Examples of hypertonic solutions: G 10%, G 20%, G 40%, NaCl 10%.
Changes in osmolarity are a particular threat to the brain. The rapid decline in ECF osmolarity can result in brain edema. A rapid rise in ECF osmolarity may in turn lead to the pontine myelinolysis.
_
Regulation of extracellular fluid volume and sodium metabolism
The volume of the circulating fluid is essential for maintaining blood pressure. Hemodynamic parameters conversely help to regulate the content of Na+ in the body.
Sodium cation – Na+
Sodium cation is the primary cation of extracellular fluid. Its concentration in ECF is 135-145 mmol/l , the concentration in intracellular fluid is much lower – about 10 mmol/l. Sodium cation is along with chloride cation responsible for 80 % of the ECF osmolarity – it binds the most water of all ions (therefore retention of Na+ causes water retention). The content of Na+ thus determines the content of water in the ECF and hence in IVF. Volume of intravascular fluid is critical for the regulation of blood pressure and cardiac output.
We ingest Na+ primarily in the form of salts. The recommended daily dose is approximately 2.4 g (70 mmol), which equals to 6 g NaCl. However in reality, the salt intake in developed countries is much higher, which increases the risk of developing hypertension. Na+ is excreted primarily through the kidneys (U – Na = 50-200 mmol/l) and sweat (sweat is hypotonic fluid). The kidneys have a high ability to secrete Na+.
Regulation of Na+ concentration in the body
Na+ content in the body is regulated by two main systems – the renin-angiotensin-aldosterone system and the system of natriuretic peptides.
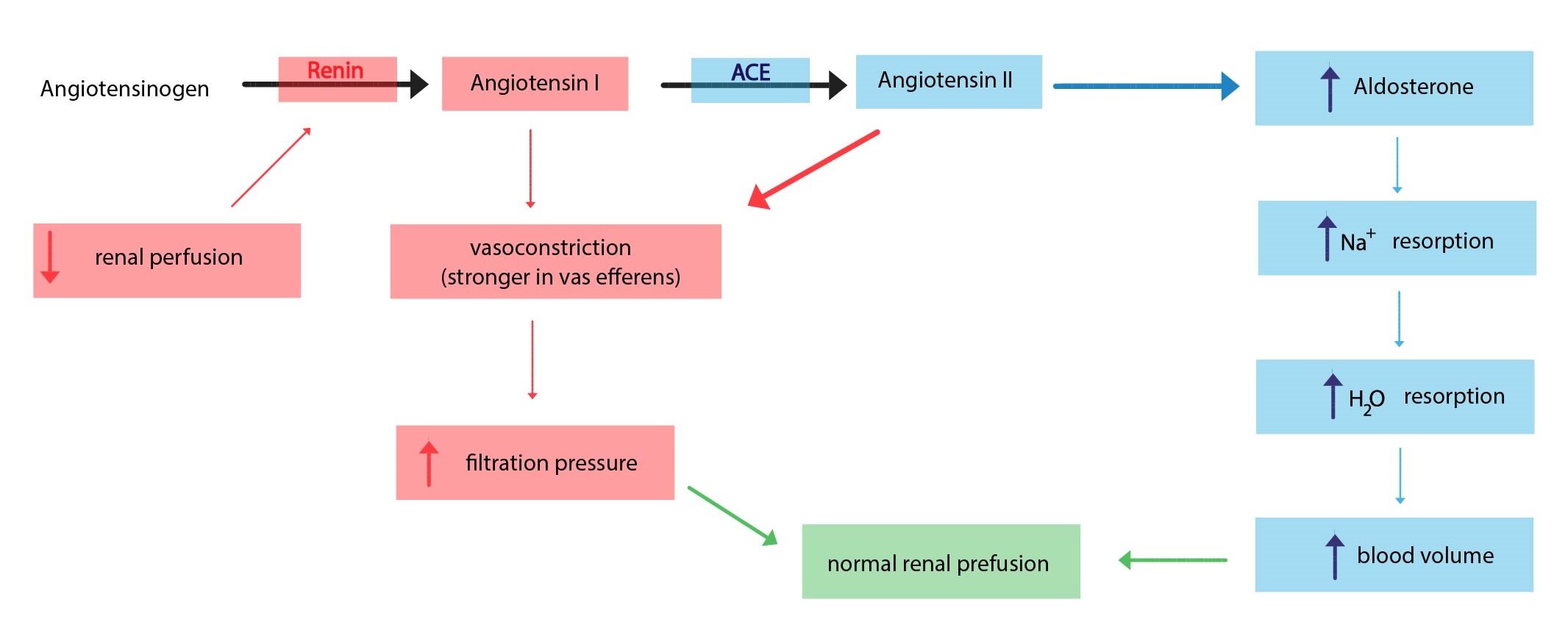
Regulation via the renin-angiotensin-aldosterone system (RAAS)
In the case of insufficient blood flow to the kidneys (e.g., decrease in blood volume) cells of the renal juxtaglomerular apparatus begin the synthesis of protein renin. Renin is an enzyme, which catalyzes the conversion of plasmatic angiotensinogen to angiotensin I. Angiotensin I is then converted by angiotensin converting enzyme to angiotensin II, which stimulates aldosterone synthesis and causes vasoconstriction. Aldosterone production is also stimulated by increased levels of serum potassium.
The main effects of aldosterone (mineralocorticoids) are:
1) Retains Na+ and water in the body – they strengthen Na+ absorption in the distal tubuli
2) Increases blood pressure by increasing in extracellular fluid volume (intravascular fluid predominantly) – related to point 1)
3) Increases urine excretion of K+ a H+ in distal tubuli
Regulation by the natriuretic peptides
At present we know of several natriuretic peptides. Two of them are considered to be principal – ANP (atrial natriuretic peptide) and BNP (brain natriuretic peptide). Both have significant vasodilating effects, increase natriuresis (they inhibit reabsorption of Na+ in distal tubuli – increase in Na+ losses to the urine) and diuresis and reduce sympathetic activity. Quite unusual is the fact that both are secreted by cells of the heart – the heart is therefore an endocrine organ.
ANP is secreted by cardiac atrial cardiomyocytes, and the stimulus for secretion is increased wall stress in the atria (e.g., increased venous return – causes expansion of the atria). BNP was first described in porcine brain (the reason for the designation). In humans , however, it is secreted primarily by cardiomyocytes in heart ventricles – the signal is increased tension in the wall of the ventricle or ventricular dilatation. Natriuretic peptides thus mediate the body’s response to excess Na+ and increased blood volume – their significant secretion occurs in response volume overload of the heart.
Clinical correlation:
In clinical practice, levels of natriuretic peptides, predominantly the N-terminal fragment of proBNP (NT – proBNP), are measured to rule out heart failure in people who present with sudden difficulty in breathing or to determine the prognosis of patients with known heart failure.
Reserves of Na+ in the body can be assessed by clinical examination. Decreased volume of ECF can result in dry mucous membranes, orthostatic hypotension and tachycardia (potentially shock). On the contrary increased volume of ECF indicate edema and lung crackles. Laboratory tests for Na+ reserves are used secondarily (S -Na+).
Na+ losses can occur in several ways – through urine, sweat or through the GIT. Some causes of loss through urine are administration of diuretics, osmotic diuresis (hyperglycaemia in DM), polyuric phase of renal failure, insufficient production of aldosterone, insensitivity of the distal of distal tubule cells in kidney to aldosterone. Losses through GIT occur during vomiting, diarrhea or through fistula and drains. Significant quantities of Na+ may also leave the body due to excessive sweating.
Metabolism of other ions
Chloride anion – Cl–
Chloride anion is the main anion of extracellular fluid. Its concentration in ECF is 97-108 mmol/l . The concentration in intracellular fluid is much lower – 3-10 mmol/l. The chloride anion accompanies the sodium cation, and together they account for 80 % of the osmolarity of the ECF. Cl– is of great importance for maintaining acid-base balance – exchange for HCO3– (if losses of Cl– occur, body replaces them by the bicarbonates, in the retention of Cl- bicarbonate levels decrease). Yet HCl is a much stronger acid than H2CO3. Therefore, with Cl- losses metabolic alkalosis occurs and, vice versa, the retention of Cl– results in metabolic acidosis. For details, see subchapter about acid-base balance. Our immune cells can utilize the H2O2-myeloperoxidase-Cl– system to aid in the destruction destruction of phagocytosed microorganisms.
Chlorides are primarily ingested in the form of salts – therefore they are received with an equimolar amount of Na+. Likewise, chlorides are excreted along with Na+ (kidneys reabsorb them along with Na+).
Hypochloremia can result from the following conditions: vomiting, collection of gastric juice or during excessive sweating.
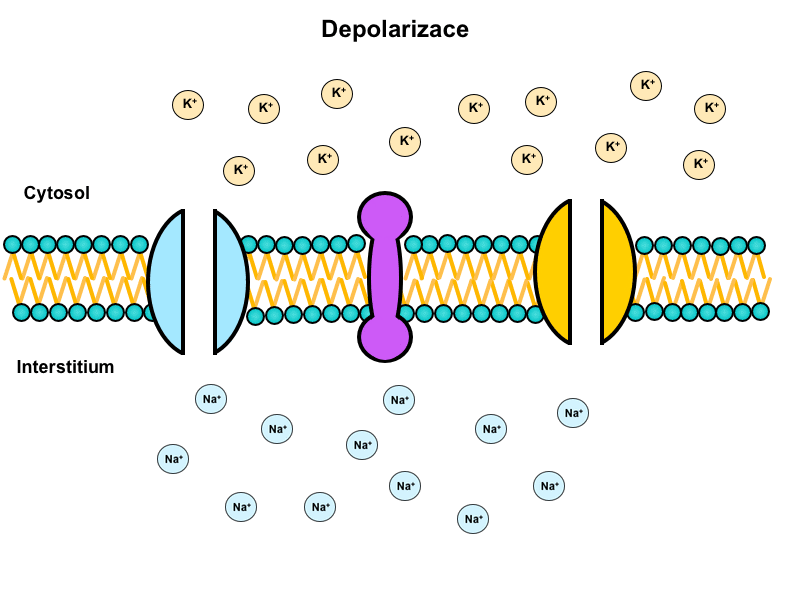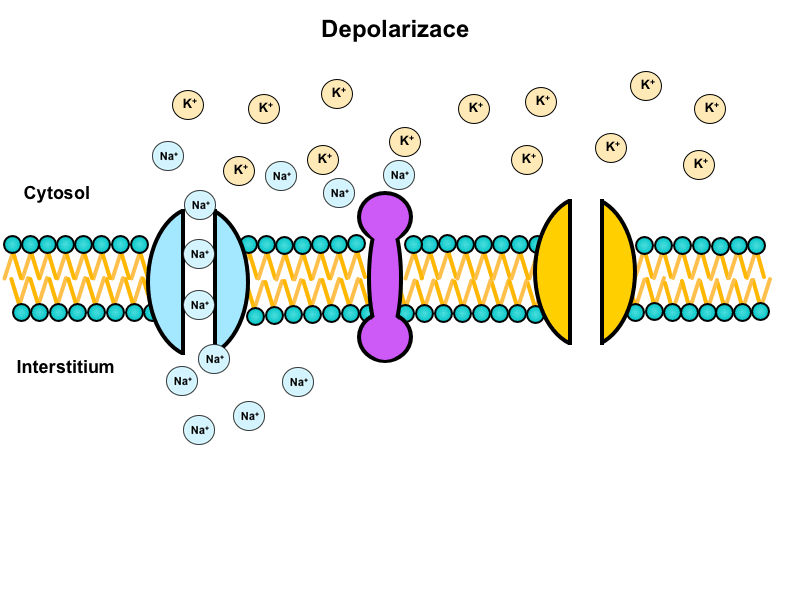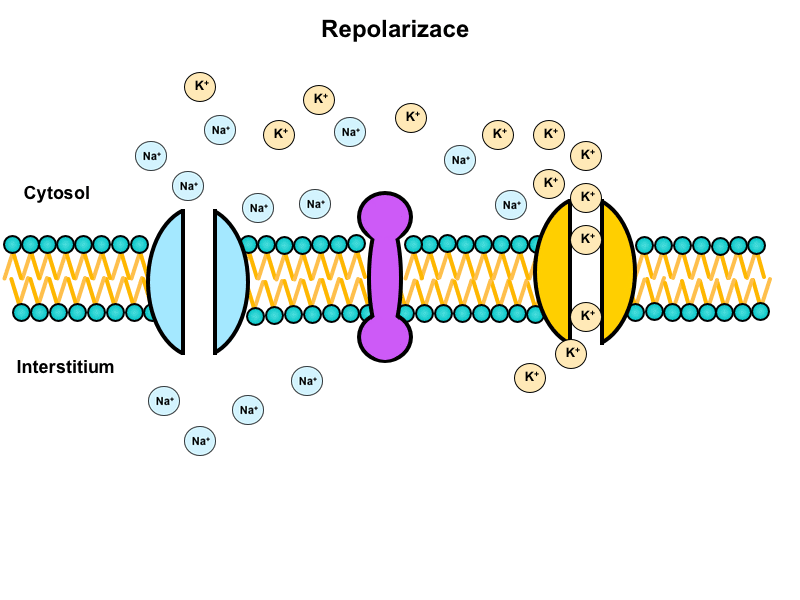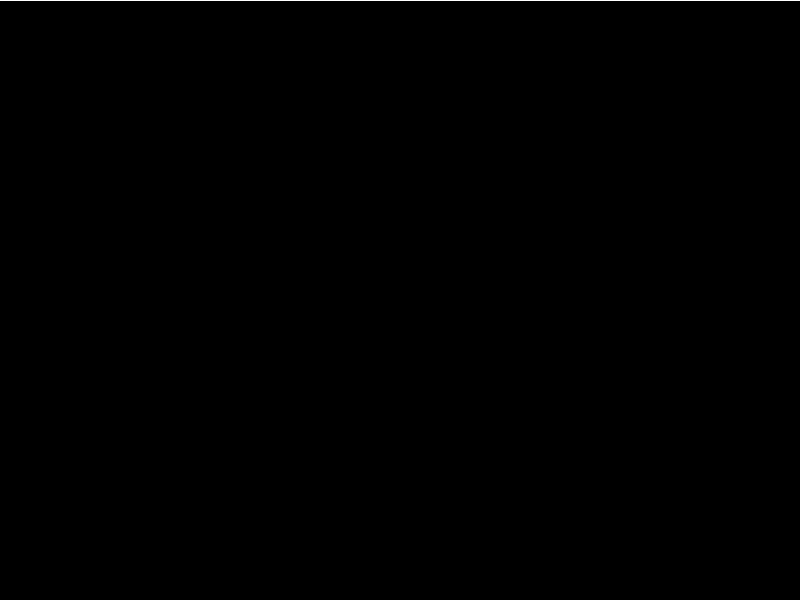
Potassium cation – K+
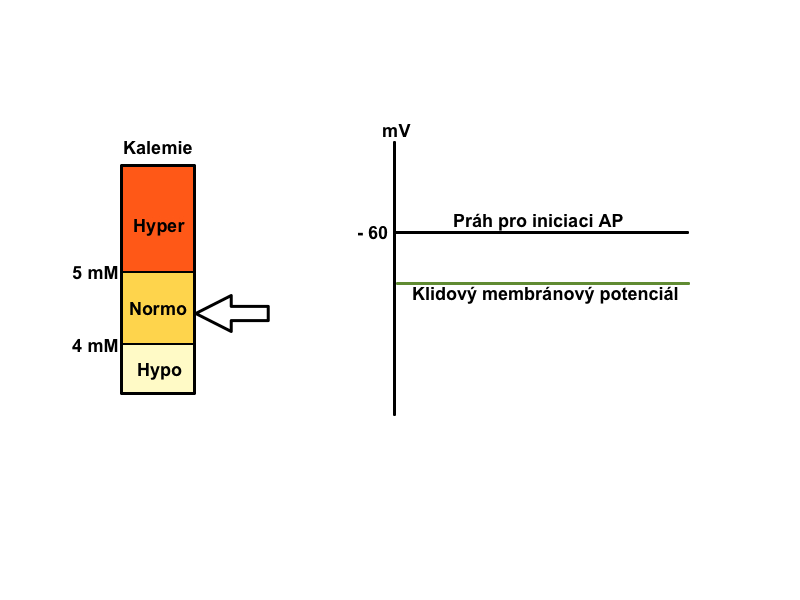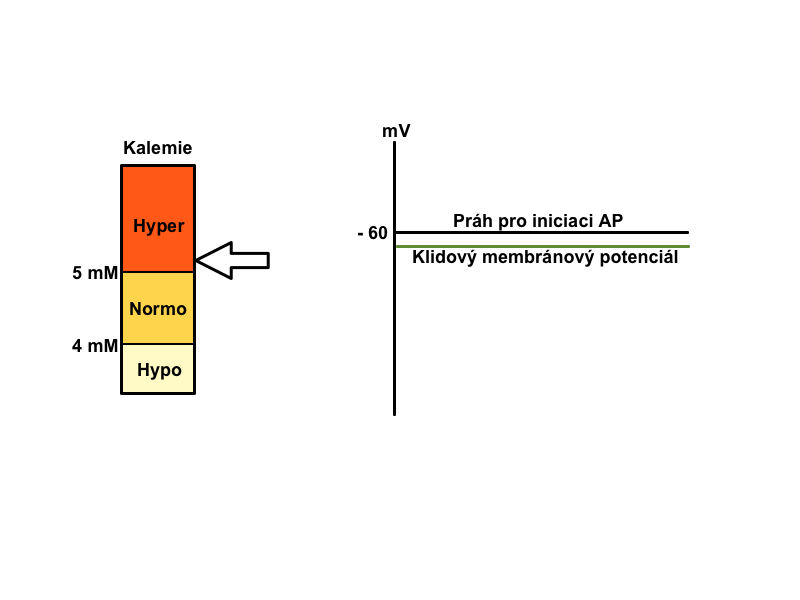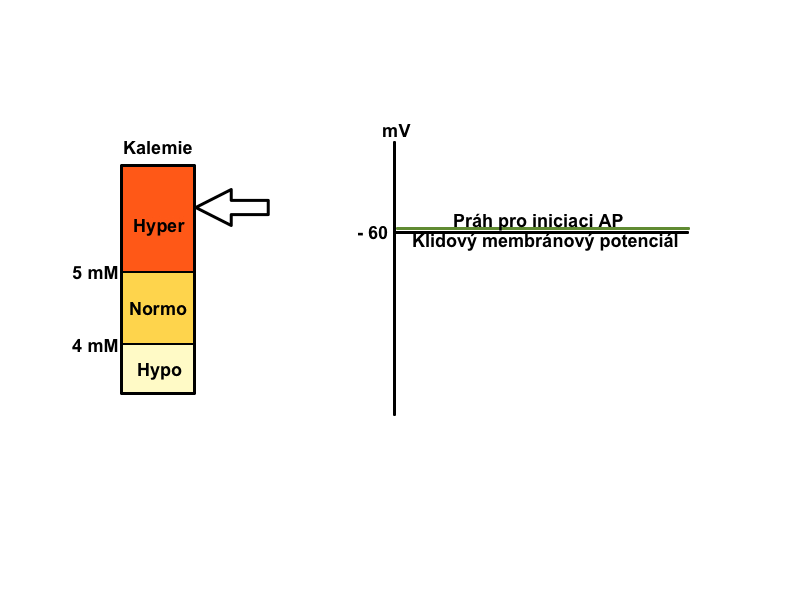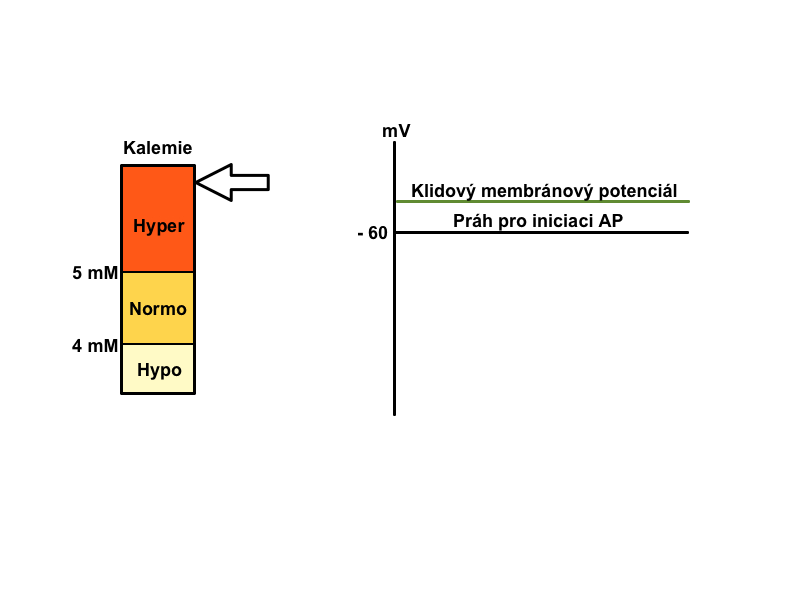
Potassium cation is one of the main intracellular cations as is the magnesium cation. 98 % of K+ is in the intracellular fluid – concentration of ~ 155 mmol/l , only 2 % remains in the ECF – concentration of 3.8 to 5.2 mmol/l. From this distribution it is apparent that the plasma levels provide us very limited information about the state of body reserves of this ion. A change in the S-K+ occurs only when the amount of K+ in the body is above 100 mmol. The shifts in potassium levels show predominantly in its changed distribution – doctor should monitor urine and waste balance.
Every day we ingest about 100 mmol K+ , the main source is vegetable diet (fruits and vegetables). Losses of K+ occur principally through excretion in urine – U-K+ = ~ 45 mmol/l and faeces – 12-18 mmol/day. K+ excretion through the kidneys depends on its intake and the levels of regulatory hormones – mineralocorticoids (mainly aldosterone). The majority of K+ is absorbed in the proximal tubule. Less than 10% of K+ gets to distal tubuli – here is the primary spot of regulation – Na+ is reabsorbed in exchange for K+ and H+ (excretion of K+ is supported by mineralocorticoids – aldosterone). By comparing serum and urine concentrations, it is apparent that the kidneys are powerful at retaining Na+ and, conversely, at eliminating K+: S-Na+ = 140 mmol/l , S-K+ = 4 mmol/l, U-Na+ = 110 mmol/l and U-K+ = 45 mmol/l → serum ratio of Na+/K+ 32:1, urine 2-3:1.
Distribution of K+ between intracellular and extracellular fluid
Intra-and extracellular distribution of K+ is influenced, for example, by:
1) Na+/K+-ATPase function
2) pH
3) Cellular catabolism and anabolism
4) Insulin and glucose
1) Na+/K+-ATPase function
Na+/K+-ATPase functions as an antiport and for ATP consumption transmits three Na+ cations outwardly from the cell in exchange for two K+ cations directed to the cell. (For more information, see Chapter 2). When there is inadequate energy production in the cells, the transfer of ions through the Na+/K+-ATPase is slowed down – K+ remains in the ECF and the local level increases while the concentration in the ICF drops.
2) pH
During acidosis cells operate as “gigantic buffers” – they take in H+. But since they took in a cation, a different cation has to be released into the ECF – K+ is leaving the cells and their EC concentration increases. The whole process is reversed by alkalosis. For more information, see subchapter about acid-base balance.
3) Cellular catabolism and anabolism
During catabolism cleavage of intracellular proteins occurs, thus releasing previously bound potassium cations which subsequently pass into the extracellular fluid – potassium levels increase. The opposite process takes place during anabolism. Particularly threatening is rapid anabolism after prolonged catabolism, which can lead to severe hypokalemia.
4) Insulin and glucose
Insulin conditioned entry of glucose into cells is accompanied by a transition of K+ into cells. This is often used in the acute treatment of hyperkalemia when a glucose infusion with insulin is administered.
Summary of regulation of K+ in the body
1) Regulation of the distribution of K+ between ECF and ICF – is responsible for acute shifts in S-K+:
a) Energetic state of cells, Na+/K+-ATPase
b) pH: alkalosis decreases S-K+, acidosis does the contrary
2) Regulation of the K+ excretion in distal kidney tubuli – mineralocorticoids (aldosterone) increase local K+ excretion
The importance of potassium cation
Physiological distribution of cations on the membrane (Na+ mainly extracellularly and K+ predominantly intracellular) is necessary for maintaining proper cell function (neuromuscular irritability, excitability of cells of the conduction system of heart, etc). The gradient of these ions between the ICF and ECF affects membrane potential. The correct ratio of the two ions is ensured by active participation of Na+/K+-ATPase.
Changes in potassium levels – hyperkalemia and hypokalemia
Hyperkalemia
Hyperkalemia, or increased plasmatic K+, can have many causes:
1) Renal failure leads to inadequate secretion of K+ by the kidneys
2) The failure of the adrenal cortex leads to defective production and secretion of aldosterone, a hormone that lowers serum potassium levels (Addison’s disease).
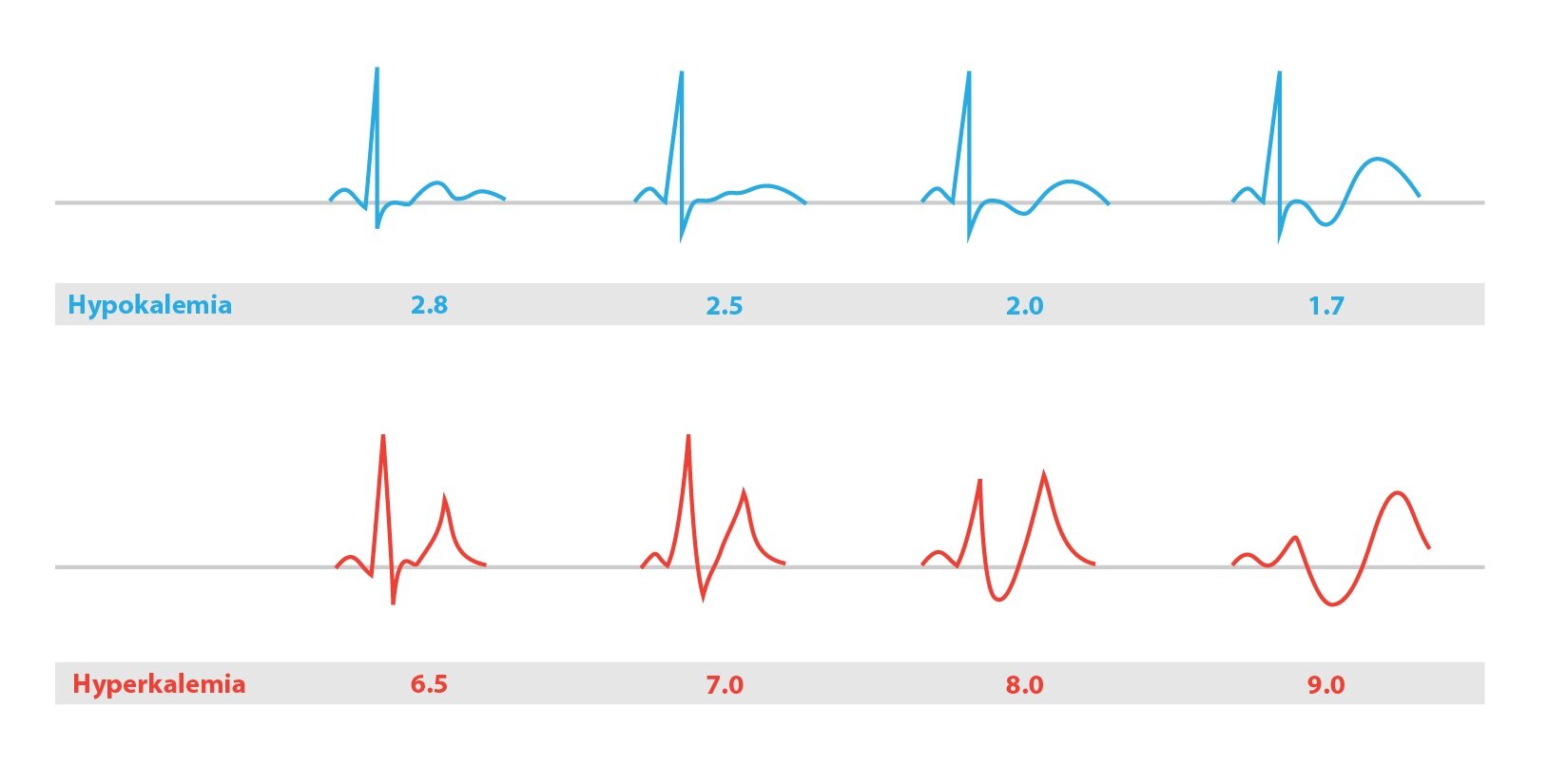
Symptoms of hyperkalemia include muscle weakness and abnormal ECG findings (see below). S-K+ values over 7,0 mmol/l are indications for hemodialysis. Higher values can be fatal, they can cause sudden cardiac arrest resulting from ventricular fibrillation (sometimes occurs even at lower values).
Hypokalemia
Hypokalemia, or decreased plasmatic K+, can have many causes:
1) Hyperaldosteronism or long-term treatment with glucocorticoids which in high quantities have similar effects as mineralocorticoids (aldosterone)
2) Diuretic overdose (furosemide)
3) GIT fluid losses – diarrhea
Symptoms of hyperkalemia include muscle weakness (up to paralytic ileus) and heart rhythm disorders.
How are potassium level shifts reflected on the ECG?
A typical finding in hyperkalemia is shortened repolarization, QT shortening, with a narrow peaked T. On the contrary, in hypokalemia the typical finding is prolongation of repolarization, prolonged QT, flat T.
Magnesium cation – Mg2+
Magnesium cation is second major intracellular cation. Its concentration in the extracellular fluid is 0.7-1 mmol/l. Magnesium has the following roles in the body:
1) Structural function in bones (2/3 Mg in the body)
2) Cofactor of 300 enzymes
3) Reduces neuromuscular excitability
Our average daily intake of Mg2+ are just units of mmol (legumes, grains, vegetables, milk).
Hypomagnesemia is manifested by muscle weakness, cramps, gastrointestinal problems and nonspecific ECG changes. It is common in alcoholics.
In the treatment of certain diseases, such as preeclampsia and other seizure disorders, we use induction of hypermagnesemia as a therapy – iatrogenic hypermagnesemia (infusion of 20% MgSO4).
_
Calcium and phosphate metabolism
Calcium cation – Ca2+
Calcium forms about 1.5 % of total body weight. The level of calcium cations in the extracellular fluid (2.25-2.75 mmol/l), is about four times higher than its intracellular concentration. 99 % of Ca2+ resides in the bones in the form of hydroxyapatite, where it serves as a mechanical support and prompt supply of Ca2+. In plasma we differentiate two basic fractions of Ca2+ – bound and free – see Chapter 5. There is a certain balance between its quantity in bone tissue and its plasma concentrations.
Importance of Ca2+
The role of the calcium cation is stabilization of membranes of excitable tissues (its absence leads to cramps), contribution to muscle contraction and coagulation – blood clotting (calcium cation is an activator of coagulation factors, whose formation is dependent on vitamin K – f II, VII, IX, X, protein C and S, and Ca2+ itself is a factor IV), and it is necessary for lactation. Calcium is also part of the inorganic bone matrix (hydroxyapatite). It is also structural component of the teeth.
Intracellular Ca2+
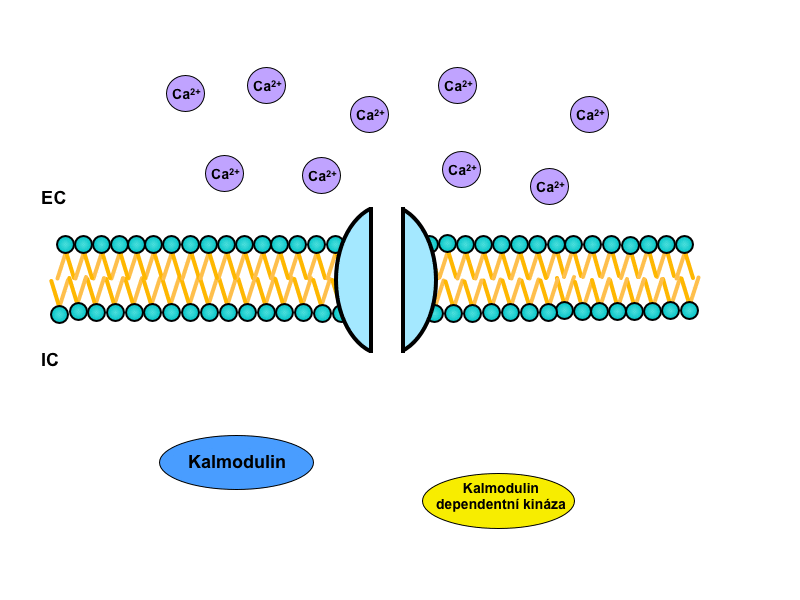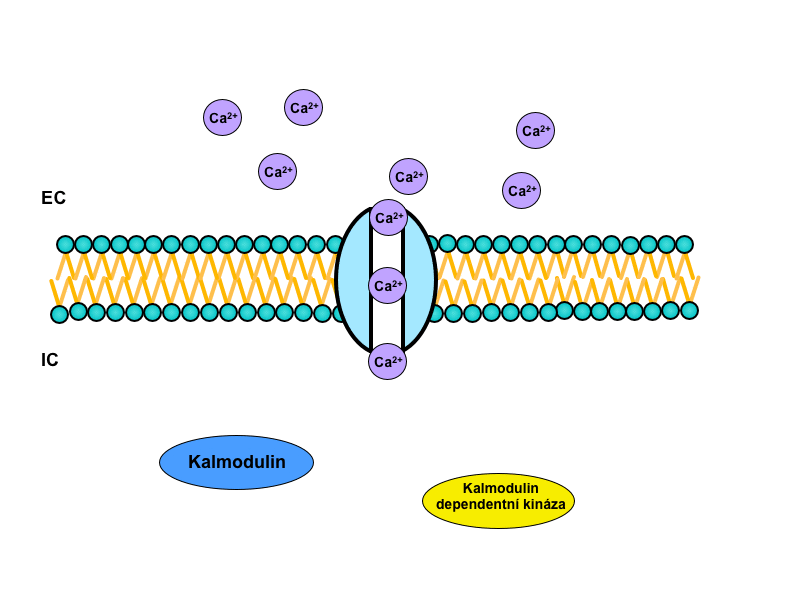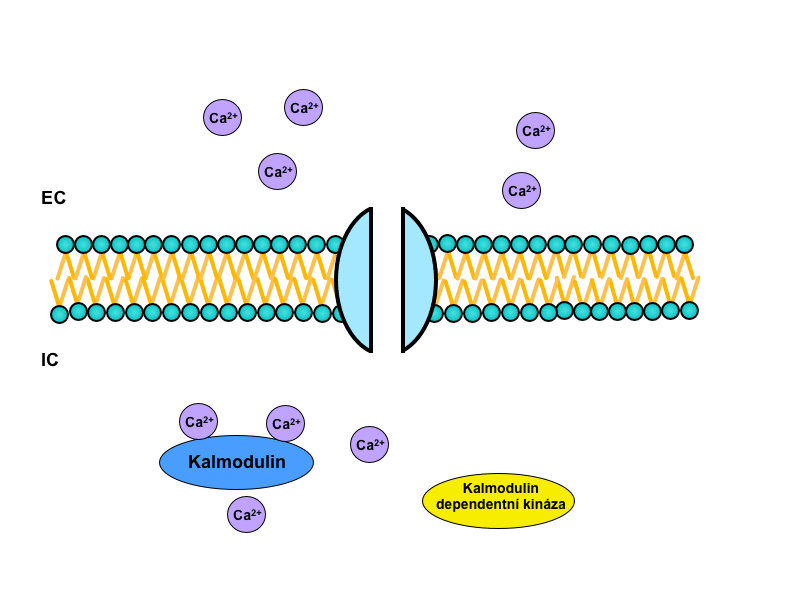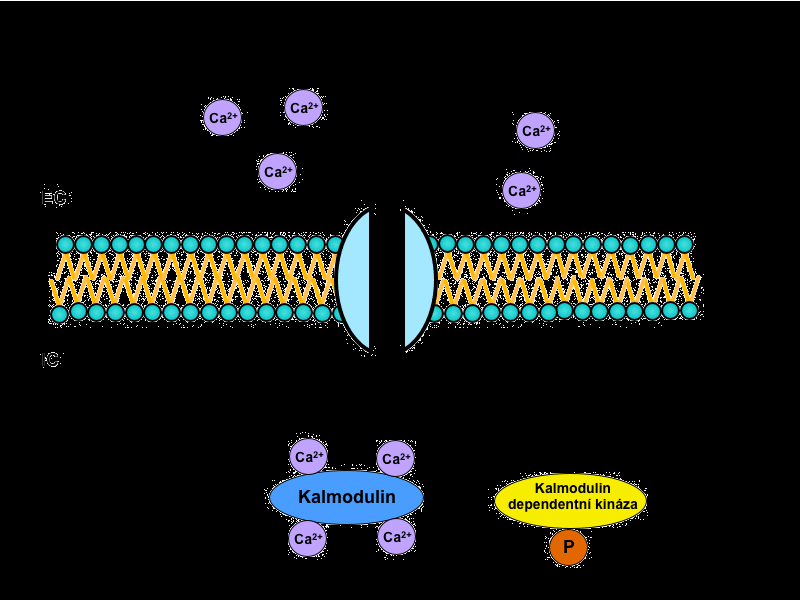
Ca2+ has the largest concentration gradient between extracellular and intracellular environments of all ions. In the cytosol there is a very low physiological concentration of Ca2+ – 10-7-10-8 mmol/l. This gradient is maintained by secondary active transport of Ca2+ for 3 Na+ and by active transport of Ca2+-ATPase. Ca2+ enters the cell through the calcium channels.
Roles of Ca2+ in the cell
Calcium ions work as a second messenger in the cell. Many of its effects are mediated through calmodulin, a major intracellular Ca2+-binding protein.
Increase in the intracellular Ca2+ concentration affects many important cellular processes:
1) The release of neurotransmitters at nerve synapses
2) Regulation of energy metabolism – activates protein kinase C, glycogenolysis, etc.
3) Regulation of muscle contraction
On the other hand, prolonged increase in intracellular Ca2+ concentration can lead to cell death.
Reabsorption and excretion of the calcium cation
The recommended daily dose of calcium for adults is approximately 1 g. The main sources are milk, dairy products and eggs. Its resorption from GIT is physiologically around 25-40 %, located primarily in the duodenum and jejunum. A specific transport protein, calbindin, is found on the apical membrane of enterocytes. On the basolateral side calcium is actively transported against the concentration gradient into the ECF.
Excretion of calcium is through urine and faeces. Ca2+ bound to plasma does not pass to the primary urine. Reabsorption takes place in proximal tubule and the ascending part of the HK.
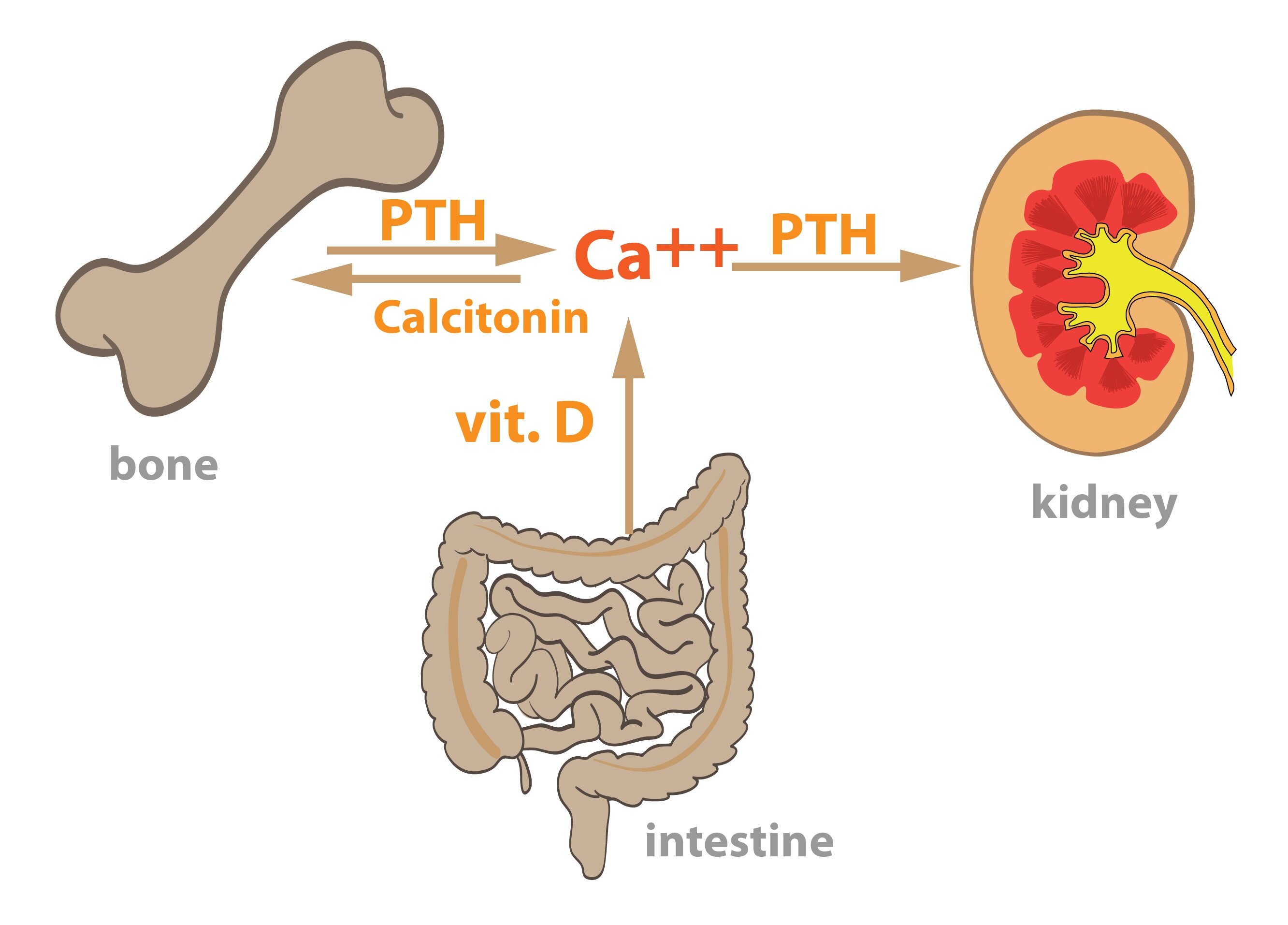
Regulation of Ca2+ a phosphate content in the body
Three hormones in the human body play a key role in Ca2+ and phosphate content regulation – parathyroid hormone, calcitriol and calcitonin.
1,25-dihydroxycholecalciferol – calcitriol (vitamin D derivative)
Vitamin D, particularly in the form of vitamin D3, is received from nutrition, but the body can also synthesize it from its provitamin 7-dehydrocholesterol. It is located in the cells of the epidermis, and photolysis with UV light forms vitamin D3. The liver can hydroxylate both dietary and synthesized vitamin D3 at position 25 and forms 25-hydroxycholecalciferol. If required by the body, this may be further hydroxylated at position 1 (the result is effective 1,25-dihydroxycholecalciferol, or calcitriol) or at position 24 (to an inactive metabolite). Enzymes catalyzing 1-hydroxylation are present in the kidney, bones and placenta.
Calcitriol stimulates protein synthesis in the small intestine allowing absorption of Ca2+ and phosphates. This ensures the availability of Ca2+ and phosphates for bone growth. It simultaneously activates osteoblasts for collagen synthesis.
Hypovitaminosis leads to defective bone mineralization, which causes rickets in children, characterized by deformation of the skull, spine, chest, and long bones. In adults, bone decalcification manifests by their softening – this is known as osteomalacia. Causes of vitamin D deficiency deficiency are not only its insufficient intake but also a lack of exposure to sunlight or kidney disease.
Hypervitaminosis D manifests by thirst, diarrhea and vomiting, itching of the skin and deposition of calcium salts in soft tissues (e.g., blood vessel walls or in the kidneys).
For more information about vitamin D and calcitriol see Chapter 9.
Parathormone
Parathormone is a peptide hormone produced by the parathyroid glands. It stimulates degradation (resorption) of bones by increasing osteoclast activity (stimulates the transformation of monocytes to osteoclasts). The result is an increased release of Ca2+ and phosphate from bones. PTH affects kidneys – inhibits Ca2+ excretion (increased reabsorption of Ca2+ from the primary urine) and conversely prevents reabsorption of phosphates from urine. It also supports the excretion of HCO3–. As a result, PTH increases calcemia, reduces phosphatemia and leads to mild acidosis.
Parathormone also supports the formation of calcitriol – stimulates 1-hydroxylation in the kidneys.
Calcitonin
Calcitonin is a peptide hormone produced by the parafollicular cells of the thyroid gland (so-called C-cells). It inhibits osteoclast activity (inhibits transformation of monocytes to osteoclasts), thereby reducing bone resorption and results in increased deposition of Ca2+ in bones. Concurrently, calcitonin decreases resorption of Ca2+ and phosphates in the kidneys. Both effects lead to a decrease in calcemia.
|
Calcitriol |
Parathormone |
Calcitonin |
|
| Bones | Ensures availability of Ca2+ and phosphate for bones | Osteoclast activation, stimulation of bone resorption and increase in calcemia and phosphatemia | Inhibition of osteoclasts, reduce bone resorption, deposition of Ca2+ into bones |
| Kidney | Slightly reduces the excretion of Ca2+ | It reduces the Ca2+ excretion (increased resorption), increases excretion of phosphate (prevents resorption), stimulates the production of calcitriol | Decreasing bone resorption of Ca2+ and phosphates |
| Intestine | Stimulates Ca2+ resorption | Only indirectly through effect on the formation of calcitriol |
– |
For more information about parathyroid hormone and calcitonin, see Chapter 11.
Shifts in calcemia – hypocalcemia a hypercalcemia
Hypocalcemia
Hypocalcemia can have these causes:
1) Hypovitaminosis D or hypoparathyroidism
2) Chronic kidney failure
Damaged kidneys fail to form calcitriol (1-hydroxylation) – reduced intestinal absorption of Ca2+. Due to inadequate excretion of phosphates, there in a phosphate accumulation in the body that causes even higher disbalance in Ca/P ratio.
3) Malabsorption
The decrease in extracellular Ca2+ concentration can lead to cramps (Ca2+ stabilizes cell membranes, thus increasing neuromuscular irritability).
Hypercalcemia
The causes of hypercalcemia include hyperparathyroidism or bone diseases (e.g. tumors). Its symptoms are polyuria, somnolence, muscle fatigue and constipation. It can lead to cardiac arrest in systole.
Phosphates
The human body generally contains about 700 g of phosphates. About 80% is in the bones and teeth. Phosphates (serum concentrations of 0.7-1.5 mmol/l) perform many functions in the body:
1) Constituent part of osseous tissue and teeth
2) Contained in vital organic compounds – phospholipids, ATP, nucleic acids, phosphorylated carbohydrates.
3) Buffer
Reabsorption and excretion of phosphates
We receive daily through our diet 800-1400 mg of phosphates, from which 60-80 % is absorbed in the intestine.
Phosphates are freely filtered into the primary urine. In the proximal tubule is resorbed more than 80 %, this process is regulated by parathyroid hormone.
Shifts in phosphatemia – hyperphosphatemia and hypophosphatemia
Hyperphosphatemia can have the following causes – kidney failure, hypoparathyroidism, vitamin D intoxication. Hypophosphatemia has these causes – hyperparathyroidism, hypovitaminosis D.
Subchapter Authors: Petra Lavríková and Josef Fontana