Content:
1. Introduction to the regulatory mechanisms of the human body
2. Hormones
3. Control of hormone secretion
4. The mechanism of action of hormones
5. Functional disorders of the endocrine glands
_
Introduction to the regulatory mechanisms of the human body
The basic task of all organ systems of the human body is maintaining homeostasis. To accomplish this task, increasingly sophisticated integration systems that regulate the function of individual tissues and organs had developed during evolution. This allowed increasingly complex organisms to achieve homeostasis by coordinated activities of these systems.
In humans, this function is served on one hand by the nervous system which is able to register immediate changes in the external and internal environment and react quickly and, on the other hand, it is served by a number of glands with internal secretion. Their reaction is usually slower, but it causes long-term changes. These two systems are closely linked to a variety of interactions that are together referred to as the neuroendocrine system. The immune system also becomes involved in situations where homeostasis in compromised.
In this chapter, we will focus only on one part of the integration plan of the human body and the endocrine glands. First, we will explore in detail the general principles of endocrine regulation in the human body.
_
Hormones
Endocrine glands affect the function of the target tissue through mediators, which are secreted into the bloodstream and are carried by the circulation over long distances. Their target may be only a specific population of cells. For example, a hypothalamic hormone corticotropin (CRH) stimulates only corticotropes in the adenohypophysis. Or in turn they can affect a majority of the cells in the body, as we see it in the thyroid hormone thyroxine. Hormones are thus able to regulate a particular function or cause complex changes in the growth and development of the organism, or cause mood swings.
From what we see above, we can conclude that hormones are very diverse substances. The following criteria are used to determine if a substance is to be regarded as a hormone (but it should be noted that with the progress of physiology these criteria lost unmitigated validity and are rather indicative):
1) Targeted effect
2) Specific effect
3) High efficiency
Targeted effect
This means that the substance regulates the function of only certain cells, tissues or organs.
Specific effect
This means that the substance causes changes in the body that are unique and which no other endogenous substances causes.
High efficiency
This means that the substance is effective (ie, produces a focused and specific effect) at low plasma concentrations – usually picomolar, micromolar at maximum.
The effect of various hormones and properties depend on their chemical structure, on which basis they can be divided into three groups:
1) Proteins and polypeptides
2) Steroids
3) Derivatives of the amino acid tyrosine
Proteins and polypeptides
Proteins and peptides are the largest group of hormones. They produced, among other places, in the hypothalamus, pituitary, pancreas , and parathyroid glands.
Their synthesis usually begins on ribosomes of rough endoplasmic reticulum as the so-called preprohormone. It is within the endoplasmic reticulum cleaved to a prohormone and subsequently transported to the Golgi apparatus. Here it is packed into individual secretory vesicles in which in which there are active enzymes cleaving prohormone to the active hormone and inactive fragment. The hormone is stored in secretory vesicles until adequate stimulus occurs that changes the membrane permeability for calcium cations or until an increase in the intracellular concentration of cAMP occurs. These changes trigger mechanisms leading to fusion of the cytoplasmic membrane with membrane vesicles and ultimately to exocytosis of the hormone.
Hormones of the nature of proteins and peptides are hydrophilic substances that dissolve well in the blood and thus do not require a carrier. This inter alia means that the whole portion of the hormone released from the vesicles into the circulation is effective, and therefore able to induce specific changes in target tissues.
Steroids
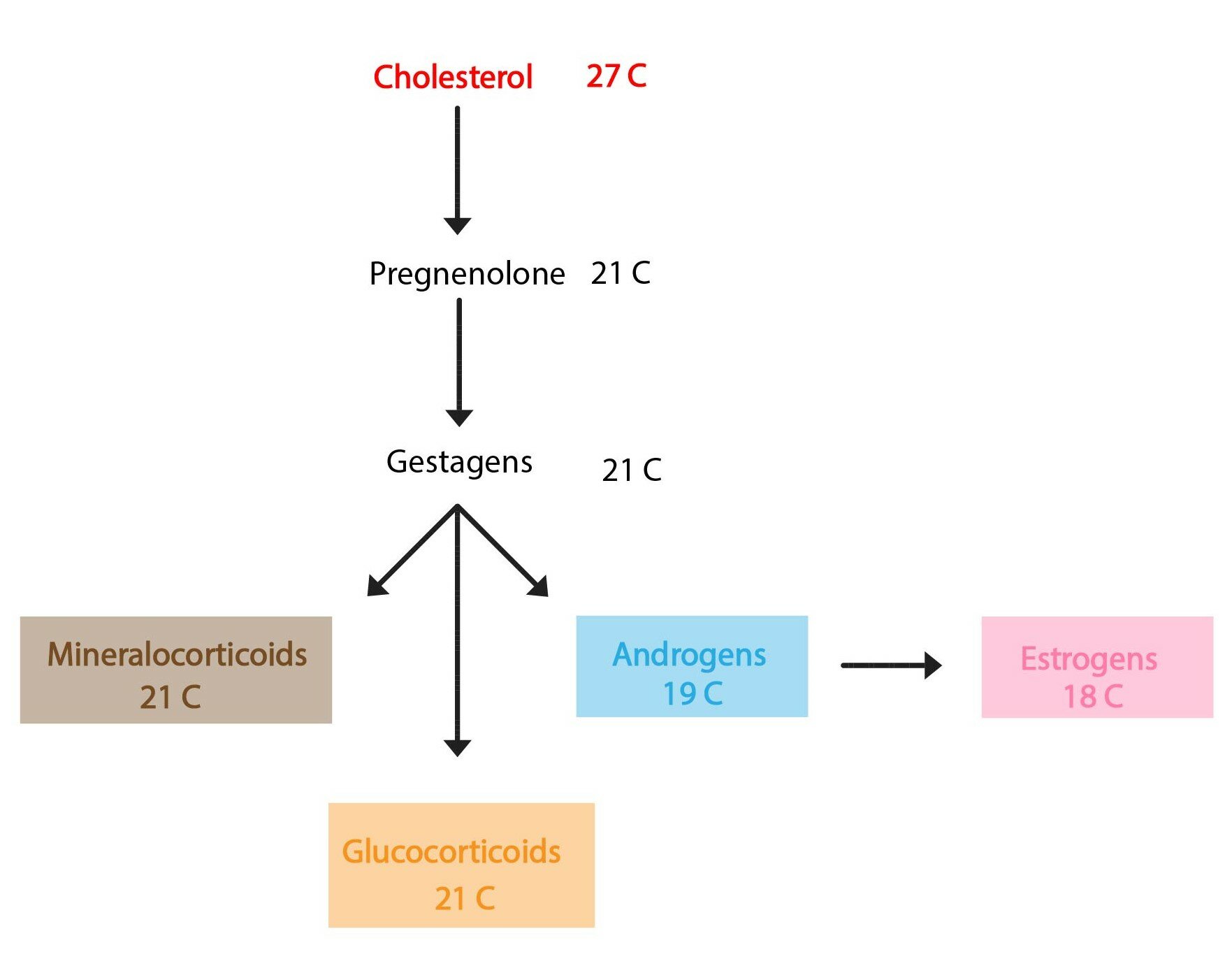
Hormones, whose chemical structure is derived from cholesterol, are produced primarily by the adrenal cortex, ovaries, testes and placenta.
Their synthesis can not be simply summarized, because it is based on a sequence of reactions specific for each hormone. We can say, however, that cholesterol is the base substrate of the synthesis of each hormone..
This creates lipophilic substances that can not be stored in secretory vesicles, because, due to their lipid solubility they would easily diffuse through membranes into the blood. Adequate stimuli therefore triggers the synthesis, rather than its secretion. It should be emphasized that in the cytoplasm of cells producing steroid hormones are found numerous vacuoles with cholesterol esters which serve as rapidly mobilizable supply of basic substrate for the synthesis.
Due to their lipophilic nature steroid hormones do not dissolve in the plasma, which necessitates transport systems that transport hormones to the target tissue. The term “transporter” usually means a protein which forms a complex with the hormone using the principle of non-covalent interactions. There are many plasma molecules, which fulfill the role of specific transporter for specific hormone (transcortin, sex hormone-binding globulin), and nonspecific plasma proteins such as albumin simultaneously participate in transport as well. Albumin has significantly lower affinity to the transported molecule than their hormone- specific carrier, but is present in high concentrations in the plasma.
These transport proteins will bind more than 90 % of the hormone. Therefore bound and free fractions of hormone occur in a dynamic equilibrium.
It is essential that the hormone – carrier complex forms a relatively large molecule which can diffuse through the capillary bed to the target tissues. The bound fraction is therefore ineffective and physiologically is only a reservoir (“pool”) of the free fraction. Only a small portion of the hormone is thus able to impart its own specific effect.
The presence of the transport system in organism offers another way to influence the instantaneous concentration of the free fraction and thus regulate the effect of the hormone:
1) The synthesis of steroid hormone
Increases the total concentration of the hormone and thus the free fraction. However, this change will be disproportionate, since the increase in synthesis rate will lead to a greater increase in bound – inactive – fraction than in the free hormone.
2) The synthesis of a specific transporter
Takes place in hepatocytes. Relatively reduces the concentration of free fraction without reducing the total concentration of the hormone. This shifts the chemical equilibrium to the side of the bound fraction.
Derivatives of the amino acid tyrosine
Hormones of this group are formed in the adrenal medulla and the thyroid gland. Despite the very specific name, it is a heterogeneous group that can be divided into two more specific subgroups:
a) Thyroid hormones
b) Adrenal hormones (catecholamines)
Thyroid hormones
They are synthesized in the follicular cells of the thyroid gland. Due to iodination these hormones have very specific characteristics, especially lipophilicity. In order to be stored in the follicles of the gland (or else they will not diffuse through the membranes), they are incorporated into macromolecules of thyroglobulin, which forms colloid. When an adequate stimulus occurs, the hormones are released of macromolecules, they are bound to plasma transport protein and set off on the path to the target tissues.
Catecholamines
Catecholamines differ from the thyroid hormones in their chemical structure (not iodated) and are therefore hydrophilic. These hormones can be stored in secretory vesicles (similar to proteins and peptides) and if an adequate stimulus occurs pre-formed hormones can be simply poured into the circulation via exocytosis. They are dissolved in blood plasma.
_
Control of hormone secretion
The concentration levels of hormones in plasma shifts based on a variety of stimuli, but it is always very strictly controlled. There are several mechanisms that contribute to this regulation:
1) Negative feedback
2) Positive feedback
3) Cyclic changes
Negative feedback
It is a fundamental control mechanism. Changes induced by a hormone in the target tissue are monitored (so-called control variable) and they they cause reverse inhibition of the hormone.
Thus, for example, a decrease in postprandial glucose inhibits insulin secretion or reaching adequate plasma concentrations of cortisol and corticosterone inhibits the secretion of adrenocorticotropic hormone ( ACTH) from the adenohypophysis. Similar principle is used for modulation of thyroid hormones production – hypothalamic TRH (thyrotropin-releasing hormone) stimulates the secretion of TSH (thyroid-stimulating hormone) from the pituitary gland, which stimulates cells of the thyroid gland to produce thyroxine and triiodothyronine. These hormones then cause feedback inhibition of the secretion of TSH and TRH.
In these examples, we see that the control variable is often one that is itself regulated. This prevents hormone hypersecretion, because when the target level of control variables is reached, its release is immediately scaled down to a maintenance level. This phenomenon is called simple feedback.
Another option is to monitor the consequences of the changes instead of the regulated function. As an example we can use hypothalamic hormones – liberins and statins. They cause changes in the secretion of glandotropic pituitary hormones and where reverse inhibition only occurs for the final products of the gland. A suitable example is corticotropin (CRH ), a hormone that stimulates the production corticotropes of the adenohypophysis to produce ACTH. CRH is reversely inhibited by elevated levels of cortisol and corticosterone. This relationship is only a subtype of simple feedback.
Positive feedback
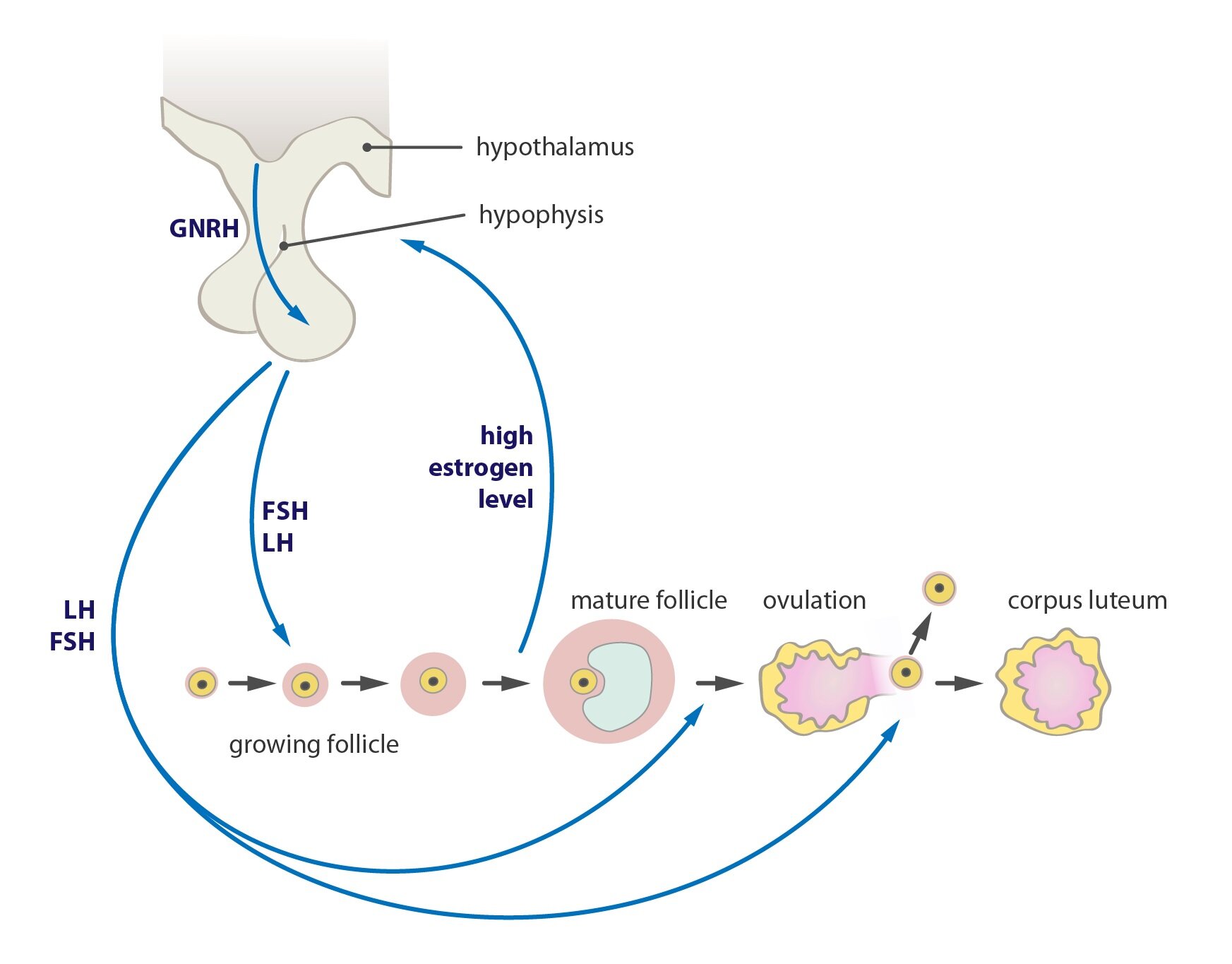
It is a special control mechanism which results in cyclic changes, each potentiates the next until it reaches the level leading to a qualitative change in the system. Here the control variable does not inhibit the production of the hormone, but rather leads to further potentiation.
A classic example is ovulation, during which luteinizing hormone synthesized in gonadotropes in adenohypophysis stimulates estrogen production in the ovaries. They again stimulate the production of luteinizing hormone until the critical concentration is reached, which leads to rupture of the follicle and release of the oocyte (qualitative change in the system).
Childbirth is another example, where we see a similar bond between uterine contractions and the flushing of oxytocin. Increase in the frequency and strength of contractions of the uterus at the end of pregnancy stimulates oxytocin secretion and this in turn affects the frequency and strength of contractions. Finally, there is a qualitative change – the birth of a child.
Cyclic changes
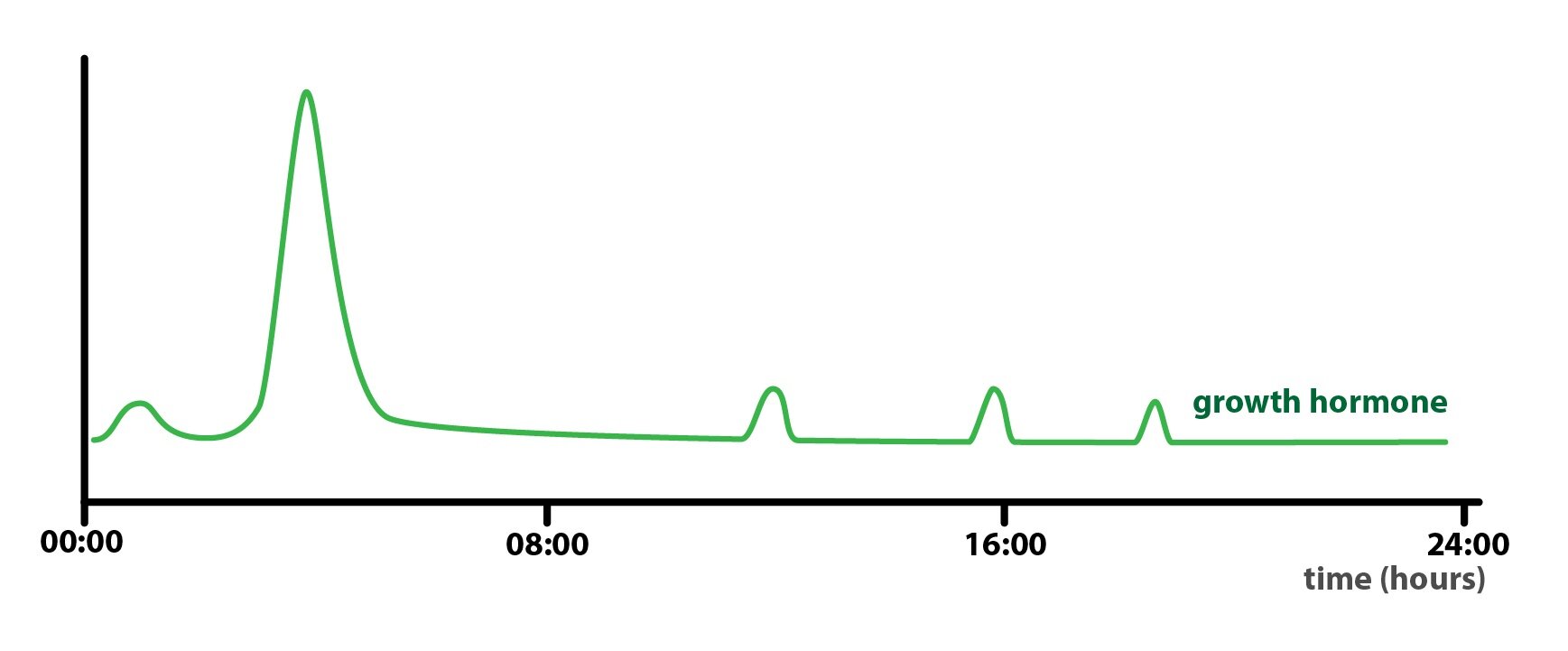
The above-mentioned regulations follow other influences as well, which can be summarized as “cyclic changes of secretion of hormones”. We can include here the effect of sleep, changing of the seasons of the year and our development and aging. These changes are usually based on the activity of the central nervous system pathways that change over time of day, cycle sleep/vigil and throughout the year.
A good example is the secretion of growth hormone, which reaches its peak during the early stages of sleep, but as sleep progresses it returns again to a maintenance level.
_
The mechanism of action of hormones
For the hormone to reach its specific effect the presence of receptors on the cells of the target tissue is required. The presence or absence of specific receptors is responsible for ensuring that the effect of the hormone is targeted. If the cell does not express the receptor, it can not respond to the presence of hormone even at unphysiologically high concentrations. Conversely if the cell has expressed the receptor, it specifically reacts even at a very low concentration.
The location of the receptor depends on the nature of the hormone. We distinguish three types of receptors:
1) Membrane receptors
2) Cytoplasmic receptors
3) Nuclear receptors
Membrane receptors
These proteins are most commonly situated in cytoplasmic membrane, which carry binding sites for proteins, peptides or catecholamines. If we look at the surface of any cell, we see tens of thousands of receptors for a variety of agents, which thanks to the fluidized nature of the membrane, “float” within the phospholipid bilayer.
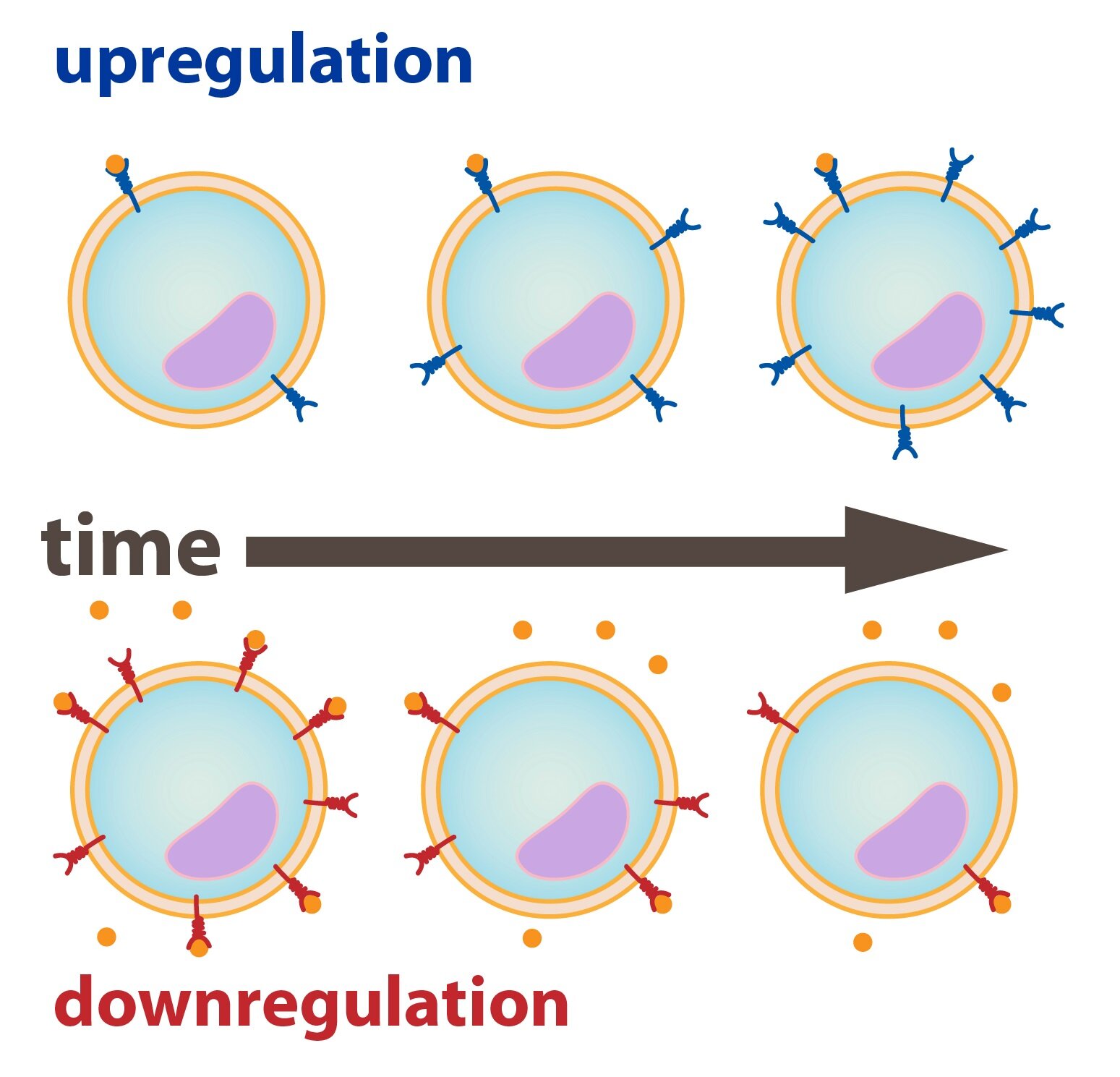
Their number of receptors is not constant. If we would observe the membrane for a longer period of time, we would see how the numbers of separate types of receptors dynamically change to accommodate the current needs of the organism – literally every minute.
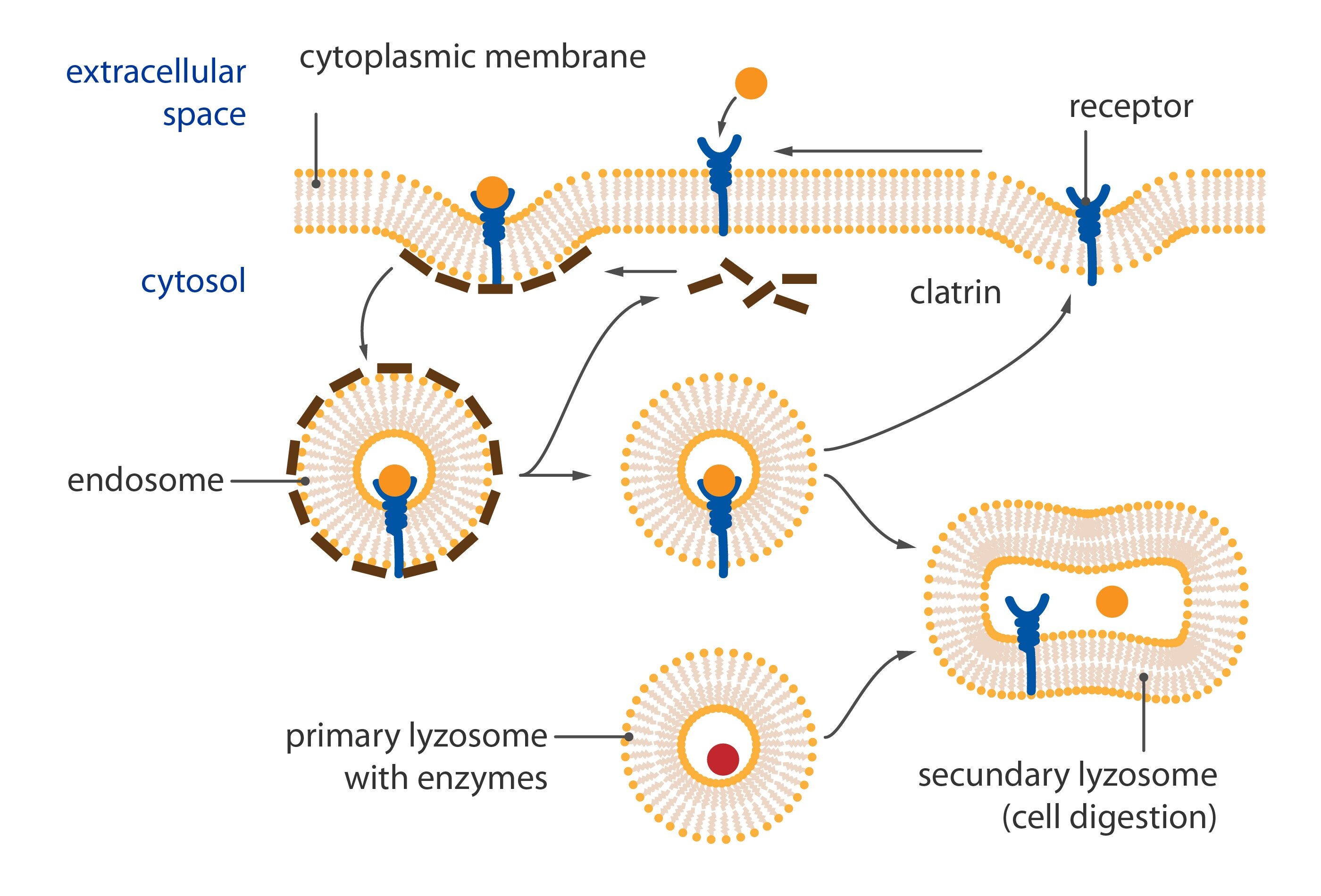
Once the hormone-receptor complex is created, it is summoned from the interstitial surface of the cytoplasmic membrane into the cytoplasm in the vacuole (or internalized). Here the receptor, depending on its type, is either reactivated (hormone molecule is thus removed from the active site and destroyed) or the vacuole fuses with lysosomes (by action of its enzymes is the entire complex destroyed and a new receptor is created).
With its own autonomous surveillance over receptor metabolism, a cell can interfere with endocrine regulation itself. The cell interferes in two ways based on the number of exposed receptors:
1) Up – regulation
2) Down – regulation
Up – regulation
If a long term reduction of the concentration of a hormone occurs in a particular tissue that is sensitive to it, its cells are able to synthesize more receptors and display them on their membrane. This will increase the density of receptors and thus sensitization of the cell. With a greater number of receptors on the membrane , only a lower plasma concentration of the hormone is necessary for an adequate response.
Down – regulation
It is a phenomenon that occurs when there are long-term elevated levels of hormones in the tissue. Depending on the type of receptor, cells will then inactivate the receptors (internalization of unused receptors) or the intracellular cascades which follow. Another possibility is a simple reduction in production.
Intracellular signaling from the membrane receptors
The signal from the activated membrane receptor is transferred to the intracellular signaling pathways. This fulfills several functions: it amplifies the signal, therefore the gradual transfer of the signal from just a few molecules of the hormone onto more and more signal molecules causes a significant change in function. Also the signal path diverges signal to several destinations, and so one activated receptor may affect many functions of the target cell. It also converges signals, and therefore two insufficiently strong signals of different origin may cause a change in the concentration of certain signaling molecules sufficient to elicit a specific effect.
Transfer from the receptor to an intracellular signaling pathway is generally threefold:
1) Ion channel-linked receptor
2) G protein-coupled receptors
3) Enzyme-linked receptors
1) Ion channel-linked receptor
It occurs much more commonly in neurotransmission than in endocrine signaling. If the receptor is coupled to the ion channel, its activation is typically achieved by the change of membrane potential of the target cell. The open channel will allow a migration of charge across the membrane. This change is immediate and leads to the activation of other channels that are opened by a change in voltage (so-called voltage-gated channels). By channel activity – those coupled with receptors, and those opened by successive potential change – the cell changes the permeability of its membrane, and in particular it changes the ionic composition of the surrounding environment.
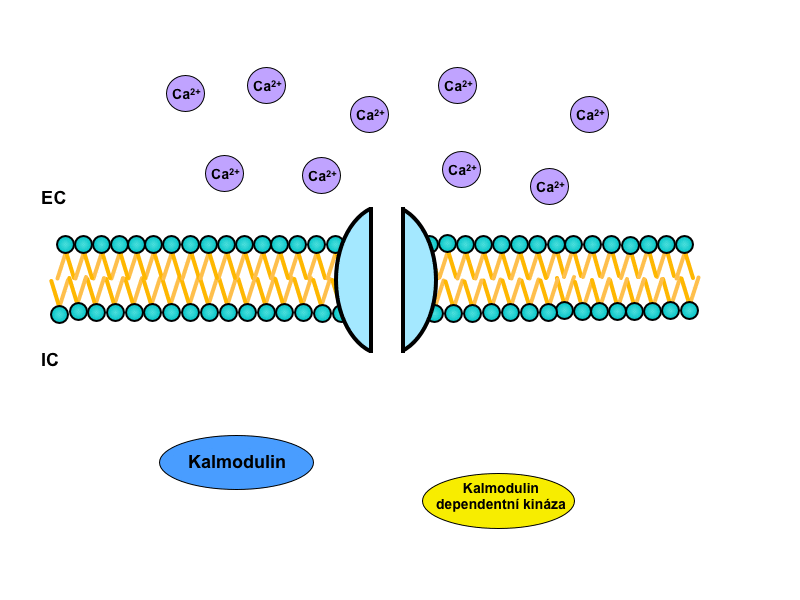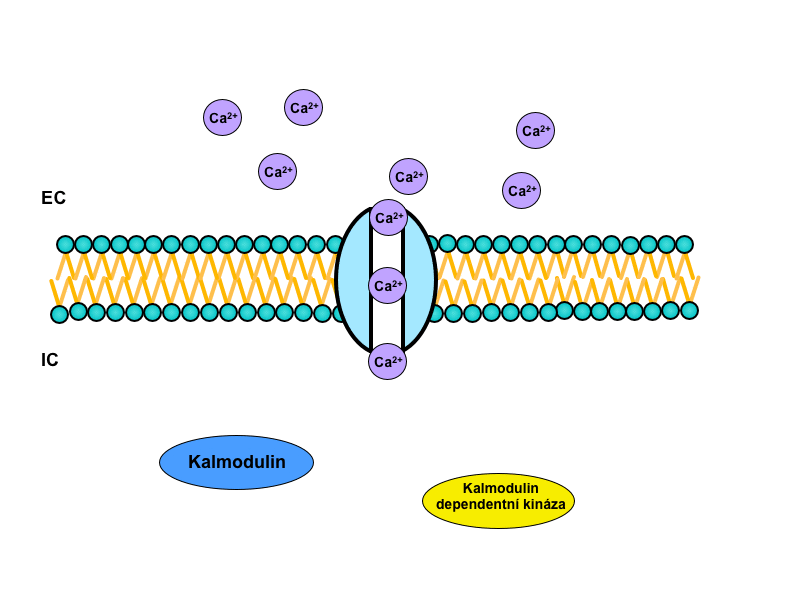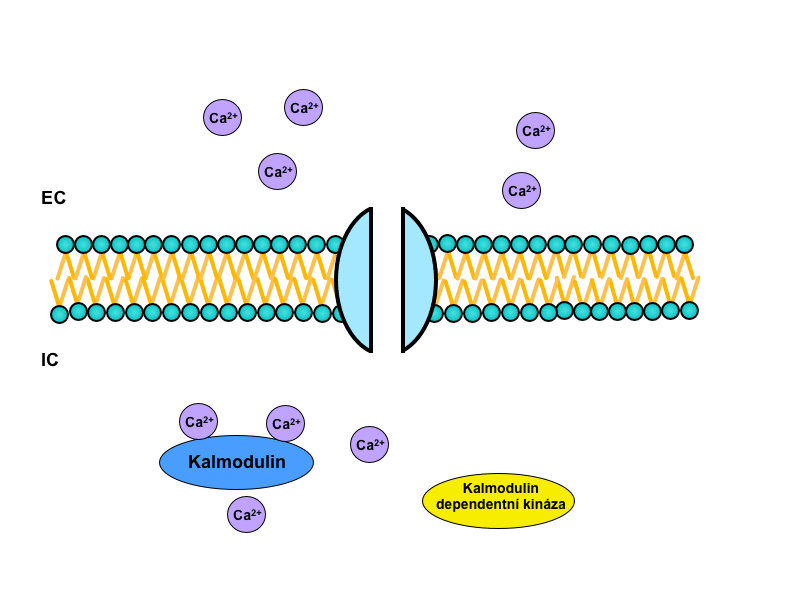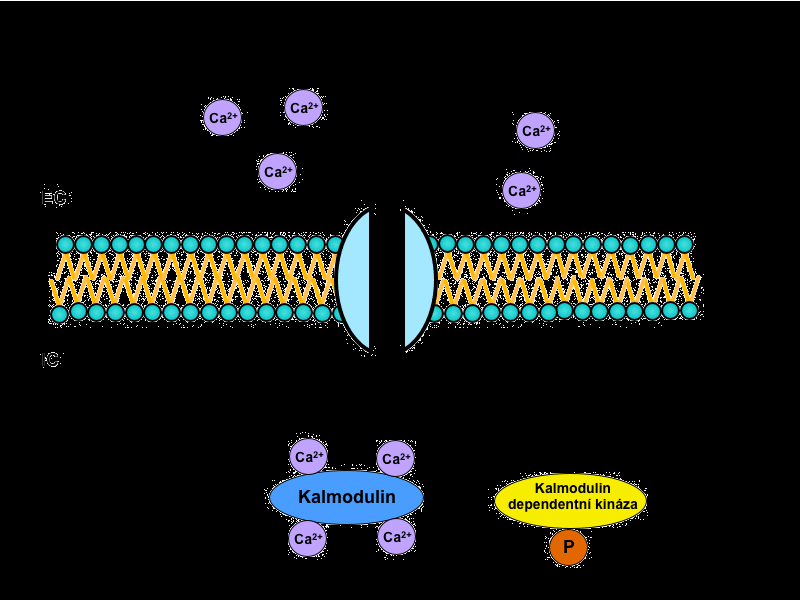
Ion, that plays a major role in intracellular signaling itself is Ca2+. The actual ionized calcium, respectively, an increase in its concentration, triggers its own calcium-calmodulin system.
The cytoplasmic protein calmodulin plays a central role in this system. It carries four binding sites for Ca2+, and when at least three of them are occupied, calmodulin changes its conformation and binds to the calmodulin-dependent protein kinases. They then trigger a signaling cascade by phosphorylation of more and more kinases, which modifies enzymatic activity. Finally – after many interconnections in the intracellular cascade – influence on the specific function of the target tissue occurs.
2) G protein-coupled receptors
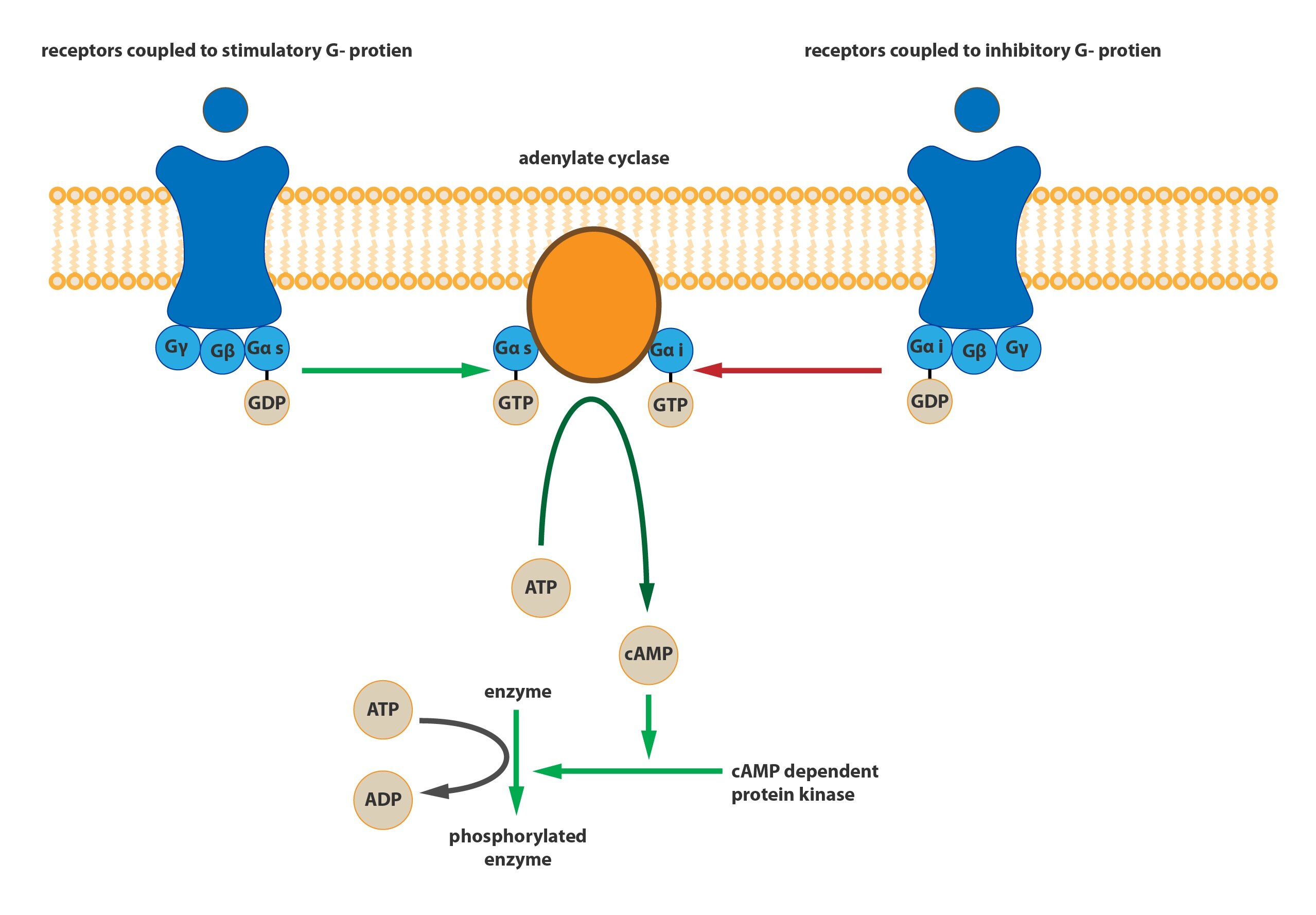
Serpentine receptors whose transmembrane domain crosses the membrane seven times, are coupled to the G proteins. It is a heterotrimeric GTP-binding protein which, in an inactive state, is associated with the receptor and is comprised of subunits alpha, beta and gamma. The alpha unit binds GDP. Activation of the serpentine receptor leads to a conformational change of G-protein, GDP is released from alpha unit and it binds GTP. This leads to dissociation of the heterotrimer. The result of receptor activation (or subsequent dissociation) is a monomer of alpha and beta-gamma heterodimer. Both products diffuse along the membrane and combine with enzymes that have binding sites for them (usually for the alpha subunit).
Based on their destination, we are able to distinguish three classes of G-proteins:
a) Gs-protein: activates adenylate cyclase, which leads to increase in cAMP concentration.
This effect alters the activity of cAMP-dependent protein kinases (depending on the kinase it may be activation and inactivation), thereby triggering a complex signaling cascade.
b) Gi-protein: inactivates adenylate cyclase, leading to a gradual reduction in cAMP concentration in the target cell.
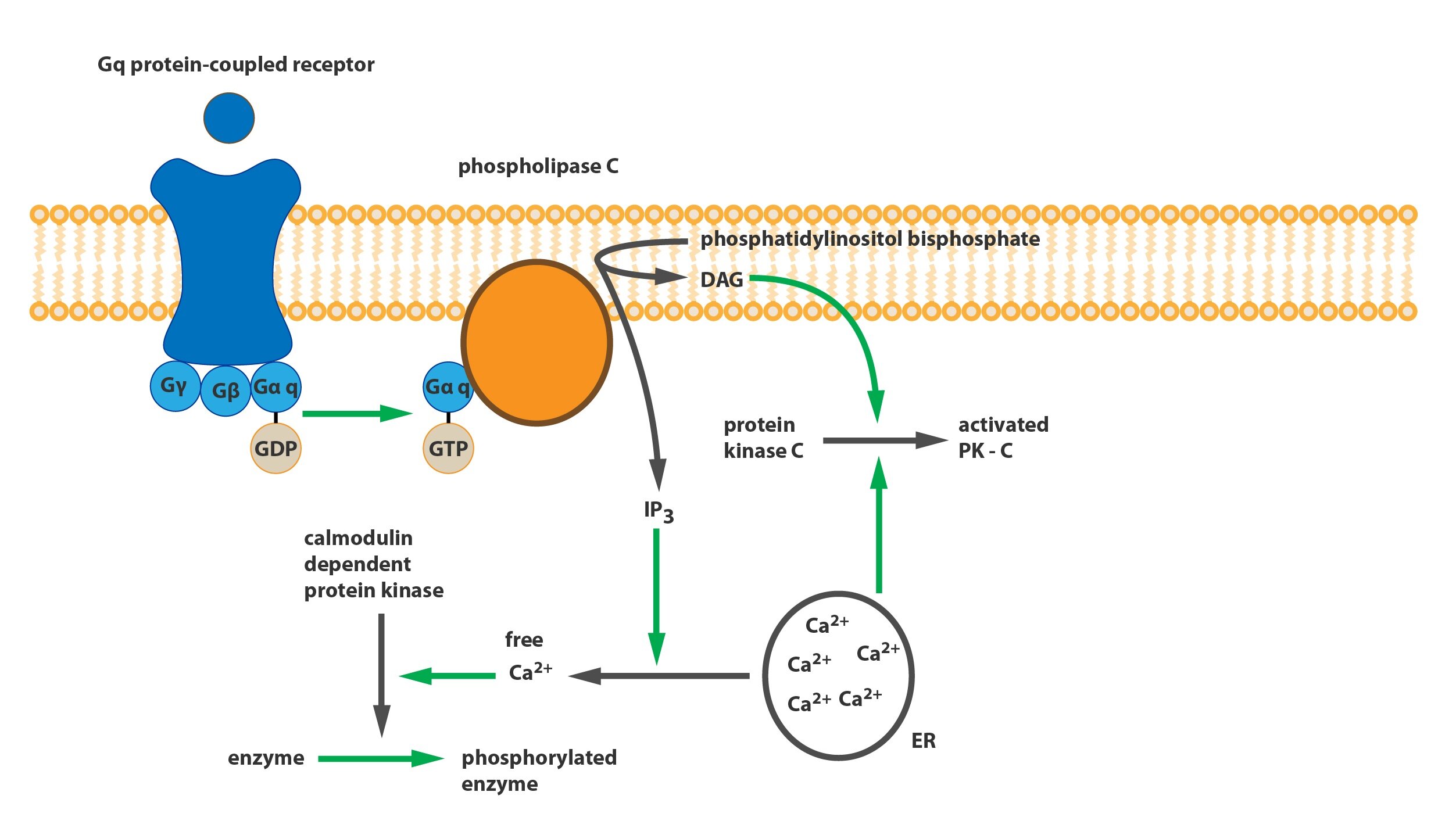
c) Gq-protein: has an effect on phospholipase C (PLC). This phospholipase then converts phosphatidylinositol bisphosphate (PIP2) present in the cytoplasmic membrane to the inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 mobilizes reserves of ionized calcium from the endoplasmic reticulum (and partly from mitochondria), which then trigger a calcium-calmodulin cascade. DAG in the cytoplasm along with calcium activates protein kinase C (PKC), which leads to the activation of another cascade of kinases.
G-protein inactivation
The alpha subunit has GTPase activity, so that after a certain time interval the GTP will cleave into GDP and inorganic phosphate. This change leads to the reassociation of monomer alpha and beta-gamma heterodimer to the original trimer coupled with the receptor.
3) Enzyme-linked receptors
Some membrane receptors are coupled with an enzyme, either in the form of an associated enzyme molecule that is activated with the receptor, or they exhibit their own enzymatic activity.
If the receptor is enzymatically active alone, it is often a kinase which phosphorylates other receptor molecules. The entire process is executed so that the activated hormone-receptor complexes homodimerize and mutually phosphorylate some amino acids (usually tyrosine or serine). This will form binding sites at the cytoplasmic domains of receptors for other enzymatically active proteins and it will trigger signaling cascades (again a chaining of phosphorylation and dephosphorylation).
Cytoplasmic receptors
These receptors are present in the cytoplasm of cells sensitive to steroid hormones which, due to their lipophilicity, pass through the membrane. The hormone-receptor complex functions here as a transcription factor that is recognized by the transporter within the pores of the nuclear envelope and transported to the nucleus. The DNA is then bound to the HRE region (hormone-responding element), which is nothing other than the promoter sequence. This mechanism changes the expression of genes, thereby activating or inactivating the transcription and, ultimately altering cell functions or creating completely new functions.
Nuclear receptors
They are functionally very similar to cytoplasmic receptors. The difference lies in the fact that they are already pre-formed at the nucleus, and therefore there is no requirement for transport of the activated receptor by the nuclear transporter. These receptors form complexes with thyroid hormones. Binding to the HRE region at the nuclear DNA alters the expression of various genes.
_
Functional disorders of the endocrine glands
Functional disorders of the endocrine glands are distinguished based on the level of hormone production:
1) Hypofunction of the gland – reduction in production of the hormone
2) Hyperfunction of the gland – increase in production of the hormone
1) Hypofunction of the gland
Reduced production of the hormone can have many causes. Among the most common are:
a) Inflammation (often autoimmune)
b) Development disorders
c) Failure of the enzymes that synthesize the hormone
2) Hyperfunction of the gland
The most common causes of glandular hyperfunction include:
a) Hyperplasia or the hormone-producing tumor (either directly in the gland, or outside – ectopic)
b) Increased stimulation by the superior glands
c) The presence of stimulating antibodies
The disorders can be according to the place of origin divided into:
1) Primary (peripheral) fault – when impairment of peripheral glands occurs
2) Secondary (central) disorders – when impairment of the superior gland occurs
Subchapter Authors: Patrik Maďa and Josef Fontana