Content:
1. Rheological properties of blood
2. Blood pressure (BP) and its measurement
3. Blood flow in different organs
4. Regulation of blood flow
5. Microcirculation
6. Lymphatic circulation
7. Venous return
8. Fetal circulation
_
Rheological properties of blood
Rheology is a scientific discipline that examines a mechanical properties of continuous media. This discipline is also called mechanics of a continuum. From the medical point of view it focuses on the physical properties of a blood.
To achieve a detailed understanding of the physiological processes in the cardiovascular system, it is necessary to understand both the biophysical properties of a blood and its interaction with the systems of blood vessels.
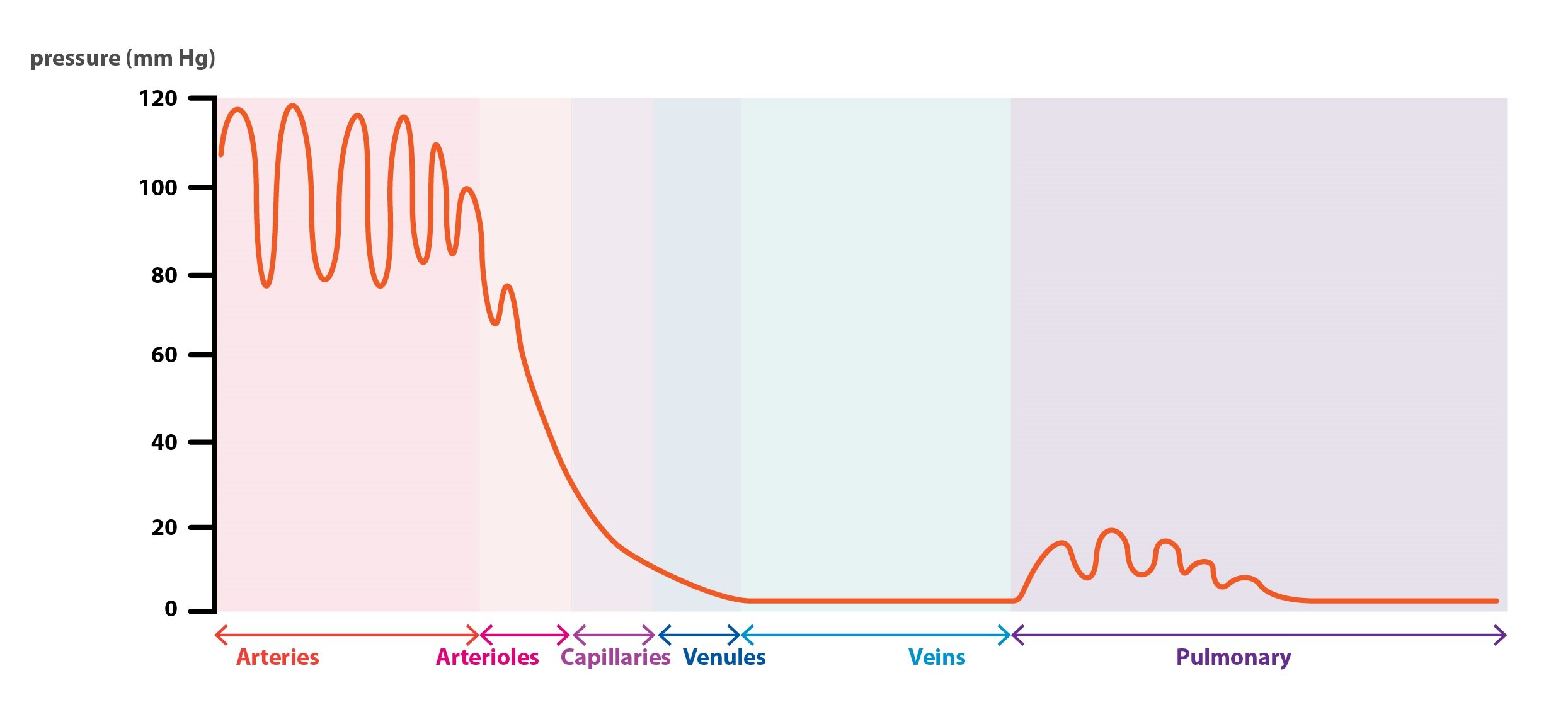
Vascular system can be divided in functional systems of arteries, arterioles, capillaries, venules and veins.
Arteries
Arteries distribute blood under a high pressure throughout the entire body. They are morphologically adapted to suit this purpose. Arteries have thick muscle-rich walls. There is about 13 % of a total blood volume in the arteries.
Arterioles
Arterioles are the smallest branches of the arteries. They role consist in controlling the flow of blood into the capillary system. This is achieved by an action of muscle cells in their walls. In particular, they attenuate fluctuating changes in a blood pressure, thus making its value constant. Action of the arterioles prevents damaging the microcirculation.
Arterioles and small arteries are known as resistance vessels, because they form a main portion of a peripheral resistance value.
Capillaries
Exchange of nutrients, electrolytes and respiratory gases takes place in the capillaries. This exchange is based on a Starling’s forces. There is only a 7 % of total blood volume in capillaries, but this portion is sufficient enough to provide nutrition to an entire organism.
Venules
Venules collect the blood from capillaries and then merge into large veins.
Veins
System of veins is often called a low pressure system or a capacitance system, due to its function as a blood reservoir. Apart from its blood reservoir function, blood flows back to the heart through veins. There is about 50 % of total blood volume in the veins.
Each of these systems must be regarded as a separate functional system, which is serially connected to the next one. To simplify a description of the events occurring in these systems, so called cross-sectional area has been introduced. Cross-sectional area is a sum of all vascular cross-sections in a specific functional system. The concept of cross-section area allow us to calculate velocity of a blood flow in a specific system.
Velocity of a blood flow is directly related to a minute blood flow in a specific system, and inversely related to a cross-sectional area:
v = F / S
If we know these three following facts:
1) Minute blood flow value that applies to the entire circulation (5 l/min)
2) Cross-sectional area of the aorta is about 2.5 square centimeters
3) Cross sectional area of the capillaries is up to 2500 square centimeters
Than it is clear that the velocity of a blood flow is about 100-fold slower in capillaries.
This relationship can be algebraically written down as:
v1S1 = v2S2
Blood flow, pressure and resistance
Blood flow is a value indicating a degree of a motion in the blood vessel. It is expressed as a unit of volume per unit of time – most commonly liters per minute (l/min). Concept of the blood flow is similar to a concept of electric current. Electric current indicates the degree of movement of electrically charged particles in the conductor. Although the similarity, there is a few differences:
Pressure gradient
Pressure gradient is a difference between the pressures at the beginning and at the end of the vessel. It is the major cause of a blood flow in the entire body. In general, liquids move from an area with high pressure value to an area with low pressure value. Thus the main function of the heart is to generate a pressure gradient.
The same principle underlines the electric current. There is an electric voltage instead of a pressure gradient. In fact, voltage is nothing else than a difference between the electric potential at beginning and at the end of a wire.
Vascular resistance
As it is true for electric current, the blood flow is inversely proportional to the resistance. Resistance of blood vessels depends on a number of factors, which will be discussed later.
Thus we can apply Ohm’s law in a case of a blood vessel as well as in case of an electric wire:
F = ΔP / R
where:
F = blood flow
ΔP = pressure gradient (P1-P2)
R = resistance of a vascular system
Blood flow
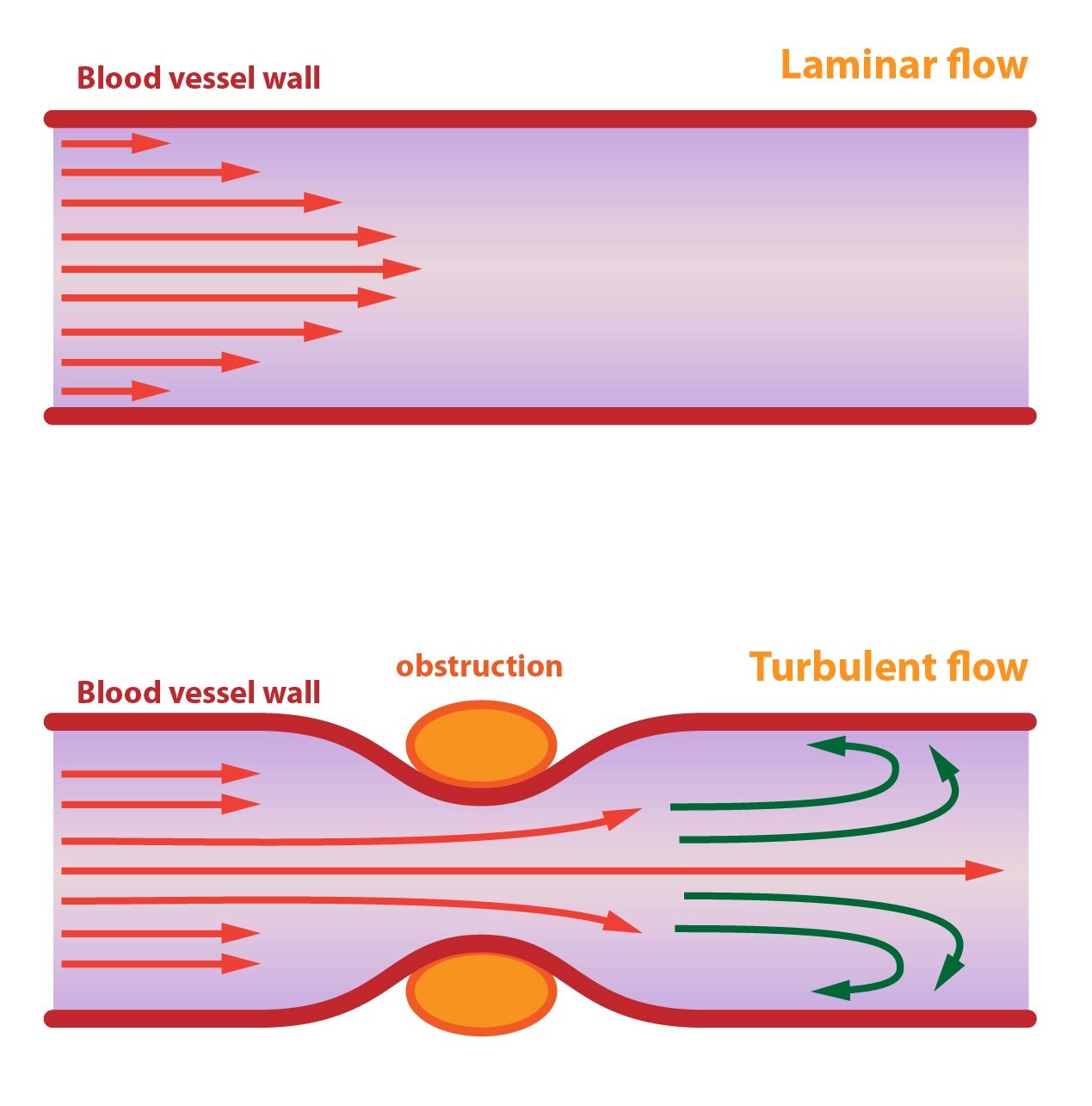
The movement of blood in the vessel can be expressed as a flux. Flux is a purely quantitative value indicating a volume of blood passing through a vessel per a unit of time. Flux is a scalar. Another point of view is to describe blood flow qualitatively. Flow can be either laminar or turbulent.
Laminar flow
Laminar flow occurs in the straight parts of arteries with intact endothelium. For every two points in the flowing volume of blood, there is a flow line. Flow lines are curves, which lie in parallel with the blood flow vector. According to the definition flow lines never crosses. Thus there are neither whirls nor vortices in laminar flow.
Laminar flow is characterized by parabolic velocity profile. This means that, there are the fastest molecules flowing in the centre of a vessel. Values of velocity decrease in a direction to a vessel surface (from centre to walls). Explanation of this phenomenon is quite simple, there is a friction of vessel wall. Molecules slowed by a contact with the endothelium are in turn slowing those flowing next to them.
Turbulent flow
Turbulent flow occurs whenever the velocity of the flowing blood is too high, the vessel is narrowed or there is an obstacle in the lumen of a vessel. There are whirls and vortices in the stream of flowing blood. If the flow become turbulent, there is a significant increase in vascular resistance and a value of blood flow decreases.
An exact way to calculate a value for which the flow become turbulent does not exist. But we can determine a probability of turbulent flow occurrence. Rate of probability is called Reynolds number.
Re = (v . d . ϱ ) / η
where:
Re = Reynolds number
v = velocity of blood flow
d = diameter of blood vessels
ρ (rho) = density of blood
η (eta) = viscosity
Re acquires following values:
1) Re < 200: Low probability of turbulent flow occurrence
2) 200 < Re < 2000: Turbulent flow is likely to occur in narrowed parts of vessels
3) Re> 2000: High probability of turbulent flow occurrence anywhere in the vessels
Vascular resistance
No direct method for measuring vascular resistance exist. It can be calculated from a pressure difference and a blood flow. It is expressed in PRU (peripheral resistance units). Vessel has a resistance 1 PRU, if pressure gradient of 1 mmHg causes a blood flow of 1 ml/s. In literature PRU is sometimes called Woods unit.
Vascular resistance is physiologically given by a variety of factors. Both friction and vascular constriction contribute. Vascular resistance is physiologically regulated by degree of constriction. Higher the constriction higher the resistance. Arteriolar constriction is the crucial factor in determination of peripheral resistance value.
Total vascular resistance
To calculate the resistance of the entire systemic circulation, we ratiocinate blood flow as cardiac output (100 ml/s). Pressure difference between the aorta and the superior vena cava is about 100 mmHg. According to Ohm’s law, total vascular resistance equals 1 PRU. This value differs according to a different physiological conditions. It can be as high as 4 PRU or as low as 0.2 PRU.
Conductance
Conductivity indicates how easily blood flows through the vessel at a certain pressure gradient. In fact, it is a constant of proportionality:
C = F / ΔP
This implies that the conductance is a reciprocal value of resistance:
C = 1 / R
Conductivity allows us to describe phenomena, where the vascular resistance would be less clear.
The velocity of the steady flow of a blood through a blood vessel is directly proportional to the pressure difference and the fourth power of the the vessel diameter and inversely proportional to the length of the vessel and the coefficient of viscosity. Thus the conductance is directly proportional to the fourth power of the vessel diameter as well. For example, if a diameter increases two-fold the conductivity increases sixteen-fold.
This principle can be algebraically expressed as Poiseuille’s law:
F = (π . ΔP . r4) / (8 . η . l)
Poiseuille’s law explain the crucial role of arterioles in regulation of a local blood flow. They are capable of up to four-fold dilatation, thus increasing a local blood flow 256 times.
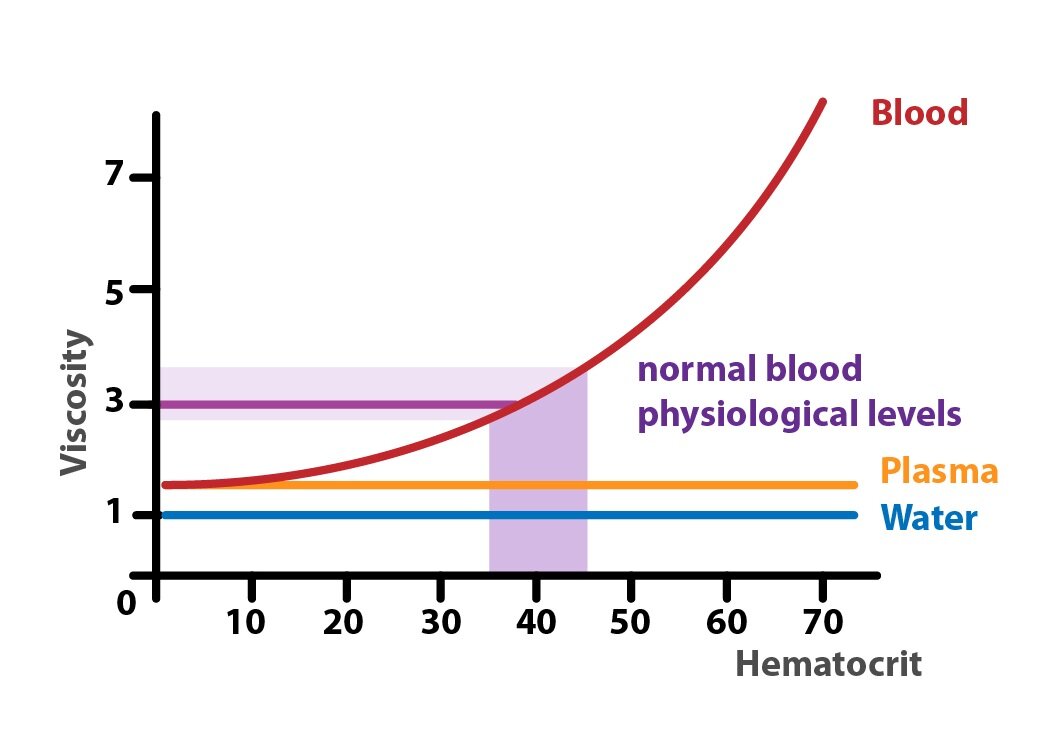
Viscosity
Viscosity is a value that characterizes an internal friction of a fluid. It primarily depends on the attractive forces between particles. Under physiological conditions, the viscosity of the blood of about three fold higher than the viscosity of water. Viscosity of blood is directly related to the number of red blood cells (hematocrit).
_
Blood pressure
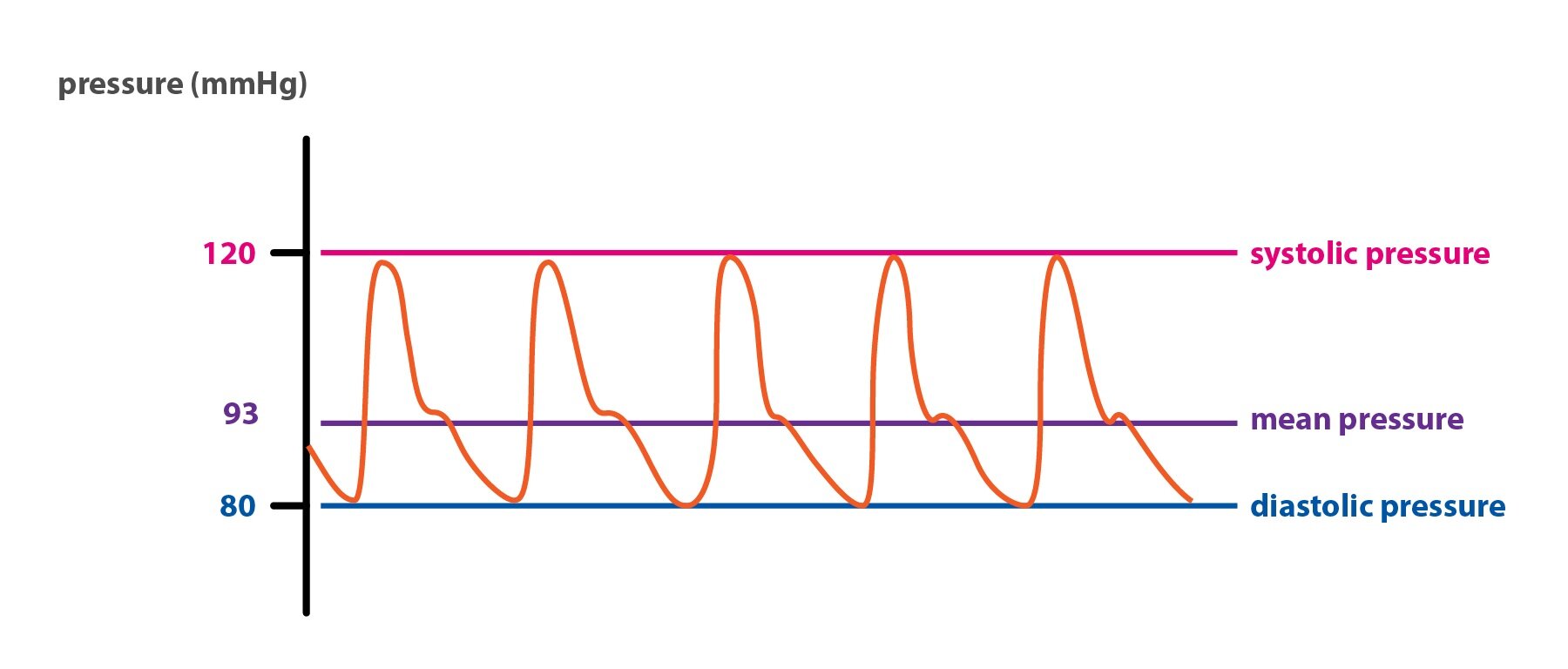
When we speak of a blood pressure, we usually refer to the blood pressure in the systemic circulation. When the left ventricle of the heart overcome the blood pressure in the large arteries, the aortic valve opens. Aortic pressure reaches its maximum, which is called the systolic pressure. Then aortic valve closes and blood pressure falls to its minimum, so called diastolic pressure. The difference between these two is known as the pulse pressure. The most important parameter for evaluation of blood flow efficiency is a mean pressure, which is an average value of pressure during whole cardiac cycle.
Measurement of blood pressure
Blood pressure can measured directly or indirectly. Direct measurement of a blood pressure is done by an introduction of the cannula to the blood stream. The most common approaches are based on introduction of cannula to a radial artery, brachial artery or femoral artery.
In routine BP measurement, there is a standard auscultation method (Riva-Rocci). An inflatable cuff wrapped around the arm, mercury aneroid manometer and a stethoscope is used. Cubital fossa is the most common location for auscultation, due to presence of brachial artery. The cuff is inflated to a pressure value just above the expected systolic BP value. At this point brachial artery closes as a result of high pressure applied by a cuff. Thus examiner hears no sound during the auscultation. Pressure of the inflatable cuff is slowly released. When the systolic pressure in the artery exceeds the pressure in the cuff, “whooshing” or pounding noises can be heard. The pressure at which these noises are first heard is the systolic blood pressure measured by the height of a column of mercury. This sound phenomenon is called Korotkoff sounds. And it indicates a turbulent flow in the brachial artery. The cuff pressure is further released until no sound can be heard, indicating the diastolic arterial pressure. Normal value of BP is 120/80 mmHg in an adult.
Pulmonary artery pressure
Due to lower resistance of the pulmonary vasculature, the right ventricle pumps blood under a lower pressure than the left ventricle does. Mean pressure in pulmonary artery is just about 15 mmHg.
_
Blood flow in the organs
Cerebral blood flow
15 % of the cardiac output enters the brain, because of high oxygen demand. Although brain represents only 2 % of total body weight, it requires up to 20 % of the total oxygen supply. Oxygen is utilized, particularly in its the gray matter.
Oxygenated blood enters the brain through two internal carotid arteries and two vertebral arteries. Vertebral arteries merge forming a basilar artery. Basilar artery and internal carotid arteries connect to each other forming a Circle of Willis. Six great arteries arises from the Circle of Willis. These supply the brain cortex, providing oxygenated blood. Deoxygenated blood is collected by deep veins that enters internal jugular veins.
Regulation of cerebral blood flow
Cerebral blood flow is strictly regulated. It must be maintained whatever it takes during all variable conditions. It is affected by the arterial and venous pressure as well as the blood viscosity and the degree of constriction of the brain arterioles.
Degree of arteriolar constriction is regulated by metabolic rate of nervous tissue. The most important factor is the rise in pCO2 (hypercapnia), which causes vasodilatation. Vasodilatation increases blood flow, thus excess CO2 is removed. Therefore fainting may occur in hypocapnia. Decrease in pCO2 causes vasoconstriction. Diminished cerebral blood flow finally results in fainting. This can be seen during hyperventilation from various causes. High plasma levels of potassium, high osmolarity or adenosine induce vasodilation as well. Local decrease in pO2 also increases cerebral blood flow. But many physiologically vasoactive substances have no effect in cerebral arterioles.
Neurogenic control is not too significant. Sympathetic noradrenergic fibers arising from the superior cervical ganglion are worth mentioning. They posses a significant vasoconstrictor effect. Parasympaticus with its mediator acetylcholine has no physiological regulatory role.
Intracranial pressure is determined by the sum of following pressures: pressure of the brain tissue, cerebrospinal fluid pressure, and the blood pressure in the brain vasculature. Enlargement of any compartment (brain tissue, cerebrospinal fluid or blood) cause significant increase in intracranial pressure. Elevated intracranial pressure compress arteries, diminishing the cerebral blood flow as well as perfusion of the nervous tissue. This phenomenon can be potentially fatal.
Cerebral blood flow autoregulation mainly involves process, where an increase in the systemic pressure causes compensatory vasoconstriction in the CNS. On the other hand, a decrease in the systemic blood pressure is compensated by the vasodilatation, which sustains sufficient cerebral blood flow.
Coronary blood flow
Coronary blood flow hear is about 250 ml/min at resting conditions. Myocardium is supplied by two coronary arteries. They arise from aorta just above the aortic valve. Left coronary artery supplies the front portion of the septum, the conduction system and the major portion of the left ventricle. The right coronary artery supplies majority of right ventricle, a portion of the septum and the posterior wall of the left ventricle. Large subepicardial branches enter the myocardium. They ends subendocardially as capillaries. Only a thin layer of cardiac muscle just beneath the endocardium is oxygenated directly by diffusion of oxygen from the blood within the chambers of the heart.
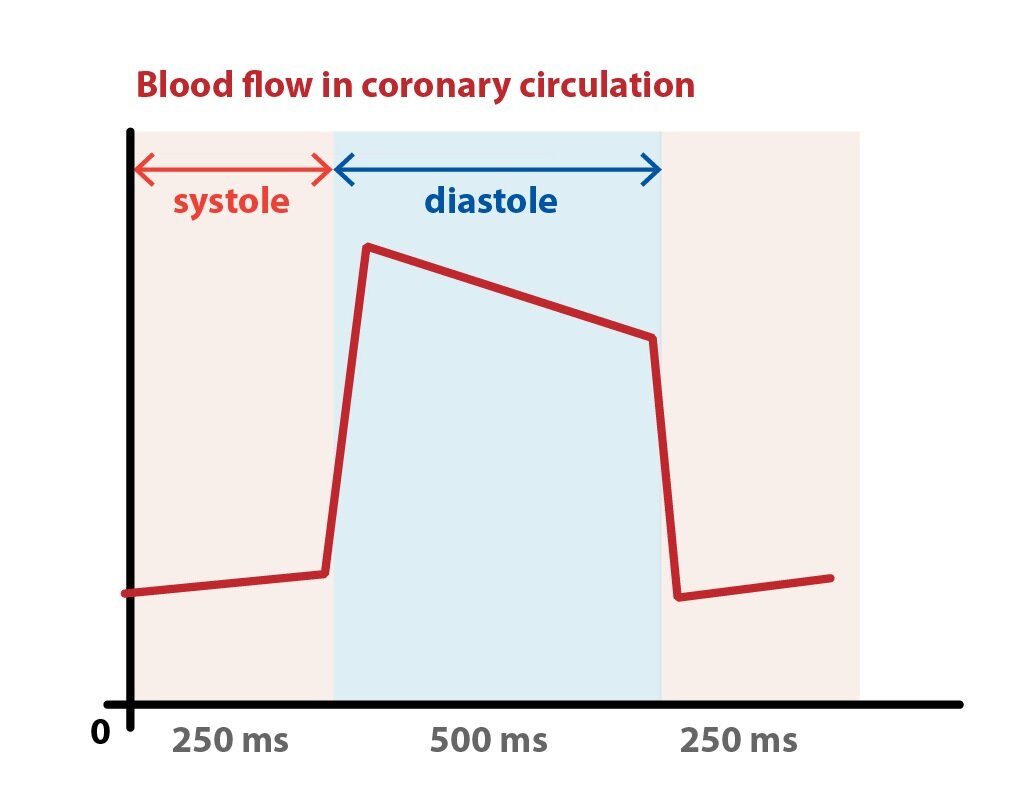
Intramural coronary arteries are compressed by contracting myocardium during the systole. The intramural pressure in this phase is so great that the flow in the left coronary artery diminishes. Direction of coronary blood flow can even be reversed. The ratio of the duration of the systole, when the blood flow diminish, and the diastole, when it reaches its maximum, is very important. If extremely high heart rate is achieved, there is a significant reduction in a duration of diastolic phase of cardiac cycle. Thus the coronary blood flow decreases.
Blood do not enter coronary arteries during the ejection phase of systole. While the aortic valve closes and isovolumic relaxation occurs, the coronary blood flow immediately increases.
Regulation of coronary blood flow
The most important regulator of coronary blood flow is pO2. The decrease in pO2 causes vasodilation. AMP cannot be converted to ATP due to decrease in oxygen level. This results in accumulation of AMP degradation product – adenosine. Adenosine is a potent vasodilator. Vasodilation is also caused by an increase in pCO2, decrease of pH, increase in extracellular potassium level, prostaglandins, histamine, NO, and many more.
Autonomic nervous system controls coronary blood flow through α-adrenergic receptors, which mediate vasoconstriction, and β2-adrenergic receptors, causing vasodilation. Decrease in blood pressure causes reflective increase in noradrenergic activity, which in turn increases coronary blood flow in the myocardium, while the skin, renal and splanchnic arteries constrict. This mechanism maintains the coronary blood flow, even when the blood flow decreases in the other tissues.
Pulmonary blood flow
100 % of cardiac output flows through the low-pressure pulmonary circulation. The pulmonary circulation is anatomically unique at the level of the microcirculation. Arterioles and venules are shorter, but have a larger diameter and richly anastomose. Mean pressure in a pulmonary artery is about 15 mmHg. Thus pressure in the pulmonary circulation is significantly lower than blood pressure in systemic circulation.
Regulation of pulmonary blood flow
Regulation of the pulmonary blood flow is mediated by a local metabolites, oxygen and carbon dioxide in particular. In the lungs they induce an opposite effects as they would in other tissues. Hypoxia as well as an increase in the pCO2 causes vasoconstriction, through the inhibition of NO production in endothelial cells. Thus blood is redistributed in way, which allows best ventilation perfusion ratio.
Splanchnic circulation
Blood flows into the intestines through the mesenteric arteries. Value of the blood flow is positively related to the metabolic activity of the gut. Blood flow increases two-fold during digestion. Blood from the stomach, bowel, pancreas and spleen flow off through the portal vein. It enters the liver and is drained from hepatic veins to the inferior vena cava.
30 % of cardiac output enters the splanchnic circulation.
There are two functionally separate circulation systems in the liver – nutritive and functional. Nutritive circulation is provided by a hepatic artery, which secure oxygen demands of the liver. In contrast, functional circulation is provided by the portal vein (blood pressure about 10 mmHg), which brings nutrient rich blood from the intestines. This nutrient rich blood passes between the hepatocyte and enters the central vein. Central veins merge into the hepatic veins, that empty into the inferior vena cava.
Liver acinus is the functional unit of the liver. It contains terminal branches of the portal vein, hepatic artery and bile duct. Blood flows from the center of acinus toward the terminal branches of the hepatic veins (central veins). Its central part (Zone I) is more oxygenated than the other zones (Zone II and Zone III). Thus Zone III is the most predisposed to a hypoxic damage.
Regulation of hepatic blood flow
Hepatic blood flow is regulated by adenosine, which is constantly produced in the liver. When portal blood flow decreases, the local concentration of adenosine rises. Increase in adenosine concentration is not caused by an increase of its production. In fact adenosine production remains constant, but with diminished blood flow, adenosine molecules aren’t washed away by a blood stream. Adenosine acts as a potent vasodilator.
Increase in sympathetic tone cause vasoconstriction.
Renal blood flow
About 25 % of the cardiac output passes through the kidneys. Most of the blood (90 %) flows through the renal cortex. Each kidney is supplied by a renal artery. This artery arises from the aorta. It divides into 2-3 branches before entering the renal cortex. One branch supplies the upper portion of a kidney, another the middle portion and the last one the lower portion. Furthermore, these branches divides into the interlobular arteries, which give rise to an afferent arterioles. Blood flows to glomerulus through the afferent arterioles. Blood is collected by interlobular veins, then passes to the arcuate veins, from those continue to the interlobar veins, which finally merge into the renal vein.
Renal blood flow regulation
Vasodilatation
Kinin-kallikrein system has a potent vasodilator effect, which causes dilatation of glomerular arterioles. This system also reduces the vasoconstrictor effect of angiotensin II. Vasodilator effect is also provided by prostaglandins (E2 and I2) and endothelial NO.
Vasoconstriction
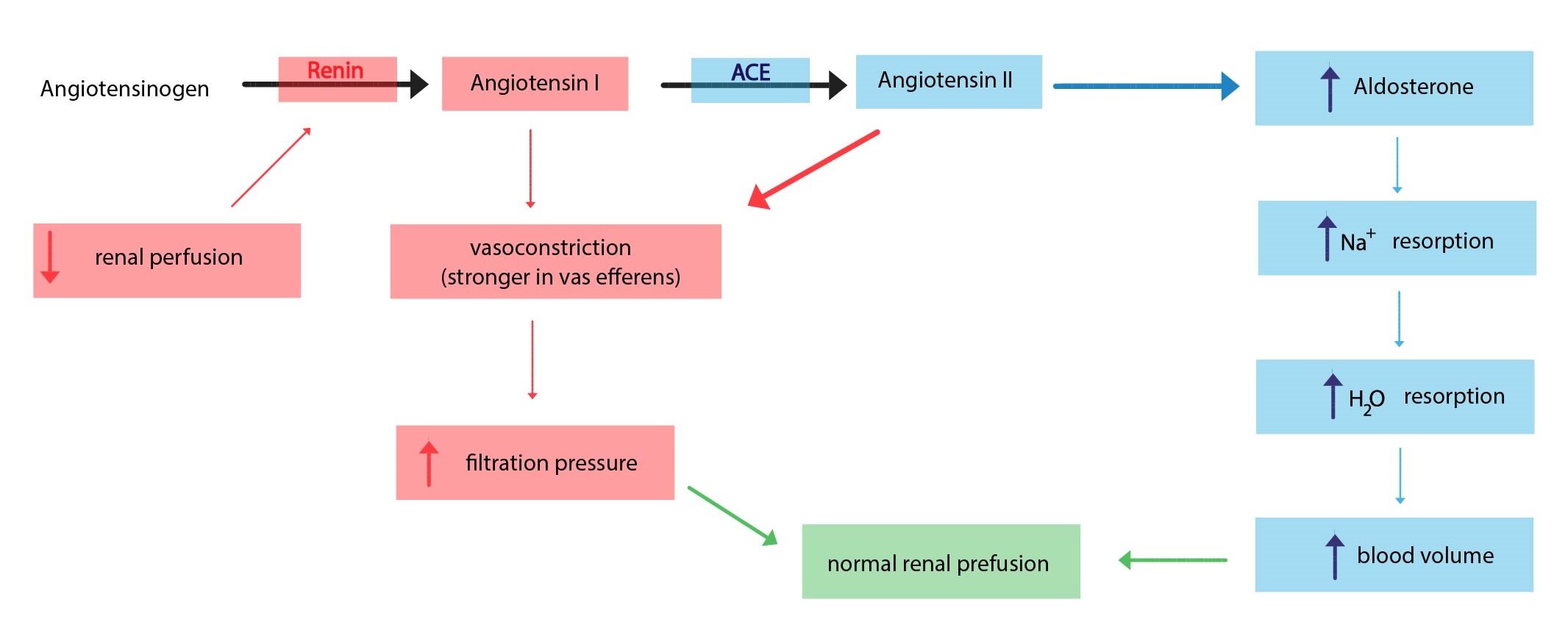
Sympathetic system causes vasoconstriction of renal arterioles, through the effect of noradrenaline and its effect on adrenergic receptors. α1-receptors posses potent vasoconstrictor effect, less potent are α2-receptors. Increase in sympathetic tone also stimulates renin secretion (via β1-receptors).
The most powerful vasoconstrictor agent is an endothelial substance called endothelin. Renin-angiotensin system also posses vasoconstrictor effect, acting in both afferent arteriole and efferent arteriole. Efferent arteriole is stimulated by an action of angiotensin II in greater extent than afferent arteriole is.
Skeletal muscle blood flow
About 25 % of cardiac output passes through the skeletal muscle under the resting conditions. But it can be as high as 90 % of cardiac output during heavy exercise.
Skeletal muscle blood flow regulation
Autonomic nervous system regulates the blood flow in the skeletal muscle. Activation of α1-adrenergic receptors (via noradrenaline) causes vasoconstriction, thus increases the vascular resistance and reduces the blood flow. Activation of β2-adrenergic receptors (via adrenaline) induces vasodilatation. Vasodilatation is also induced by local metabolites, especially the lactate, adenosine, and potassium.
Skin blood flow
Skin is one of the most important organ in thermoregulation, that human body possess. About 5 % of cardiac output passes through skin. Only sympathetic nervous system innervates skin vessels. Both adrenalin and noradrenalin cause vasoconstriction. Despite this fact vessel of the skin dilate during heavy exercise. This phenomenon is a response to an increase of body temperature. A reflex that is controlled by hypothalamic thermoregulatory centers. Local vasodilator agents include histamine and bradykinin. On the other hand serotonin and substance P are potent vasoconstrictors.
_
Regulation of blood flow
The fundamental task, that is shared by all regulatory mechanisms, is to provide an adequate blood supply to the whole organism under constantly changing external and internal conditions. This difficult task is achieved by a changes in vessel diameter.
Local regulatory mechanisms – autoregulation
Autoregulation is an ability of the individual tissues to regulate its local blood flow. According to the mechanism of action, it can be divided into myogenic and metabolic.
Myogenic autoregulation
Myogenic autoregulation is based on a reaction of vascular smooth muscle itself. Smooth muscle cells respond to an increased tension of the vascular wall by reflexive – so called myogenic – contraction. This applies to the arterioles of the kidneys, heart, gastrointestinal tract and the brain. Blood vessels of the skin and lungs lack myogenic reflex.
Vasoconstriction occurs due to activation of Na+ and Ca2+ channels in a membrane of the vascular smooth muscle. This channels are activated mechanically by simple stretching of a cell membrane. Influx of cations causes depolarization and activation of voltage gated calcium channels. This in turn causes vasoconstriction.
Metabolic regulation
Metabolic regulation occur due to changes in concentrations of certain metabolic products, such as CO2, H+, ADP, AMP, adenosine, and K+. The increase in concentration results in a dilatation of the precapillary sphincters. But there is no absolute rule. Action of these metabolites differ according to a tissue. For example, adenosine induces vasodilatation in the kidneys and lungs. Increase in pCO2 causes vasodilatation in the lungs.
Decrease in pO2 stimulates the production of vasodilator agents – prostaglandin I2 and endothelial NO. The local metabolic control is most common in vessel of the heart and the brain.
Local vasoactive substances secreted by the endothelium
Endothelial cell responds to a number of physiological stimuli by a secretion of substances that affect muscle cells of the vascular wall. These stimuli may include a stretch, friction induced by blood flow, changes in plasmatic concentration of certain hormones, substances released from the blood element (platelets, macrophages), etc. The response generally consist of increase in local secretion of vasoactive substances. Thus this effect has a paracrine character.
Nitric oxide (NO)
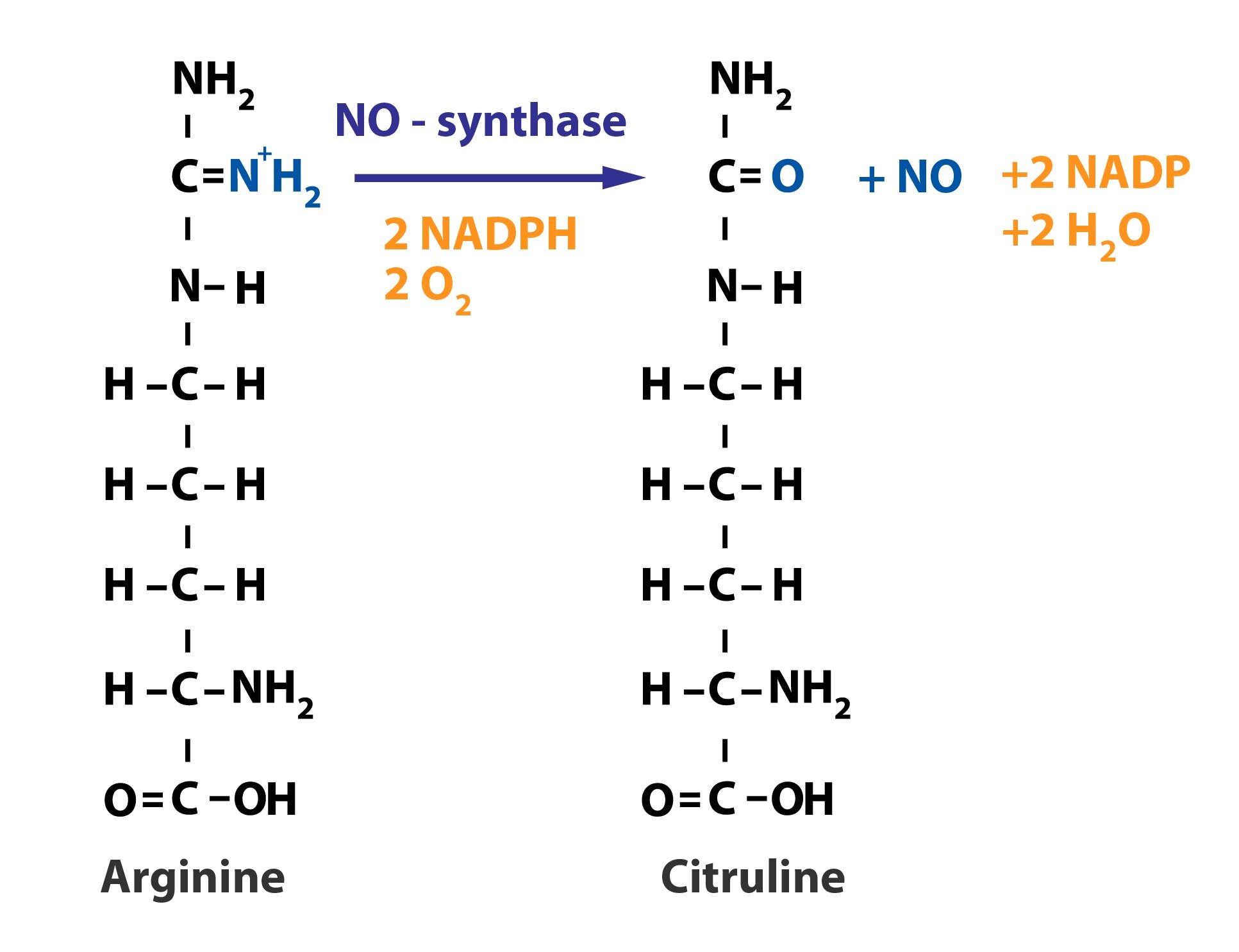
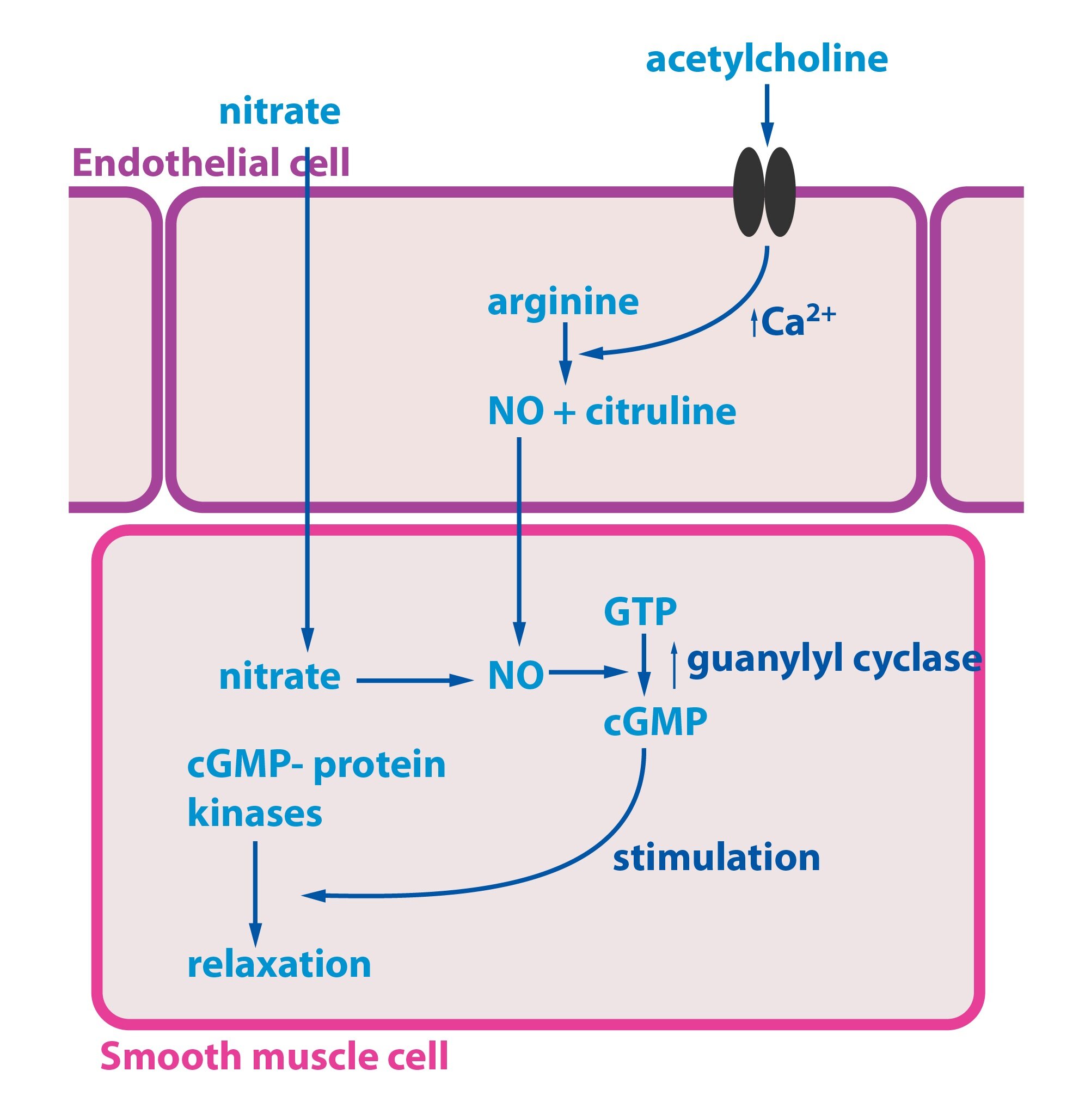
Nitric oxide is a vasodilator substance produced by the endothelium. The release may be caused by variety of factors – acetylcholine (via M-receptors), ATP, endothelin-1, histamine, and ANP. NO is synthesized from L-arginine in a reaction that is catalyzed by NO-synthase.
Now we will describe the mechanism of its action. An activation of acetylcholine receptor will start an intracellular cascade. There is an increase of intracellular Ca2+ concentration (mediated through inositol triphosphate). Increase in intracellular calcium concentration results in the activation of endothelial NO synthase. NO diffuses into the smooth muscle cells where it activates guanylate cyclase. Increase in intracellular cGMP level activate cGMP-dependent protein kinase. Finally relaxation of smooth muscle cells occur. Some medications (e.g., nitrates), are capable of entering the smooth muscle cells, where they stimulate release of NO – thus leads to vasodilatation. NO synthase is also presented in certain cells of the immune system and in some neurons.
Endothelins (ET-1, ET-2, ET-3)
Endothelins, particularly ET-1, which activates ETA receptor, is one of the most potent vasoconstrictor agents. Release of ET-1 is stimulated by an action of vasopressin and angiotensin II. ET-1 also binds to the ETB receptor, which stimulates the release of NO, and thus leads to a vasodilatation.
Eicosanoids
Eicosanoids, arachidonic acid derivatives, also posses a vasoactive effect. Prostaglandin F2a and thromboxanes A2 and B2 are potent vasoconstrictor. On the other hand prostaglandin PGE2 and prostacyclin (PGI2) are vasodilator agents.
Hormonal regulatory mechanisms
Vasoconstrictor hormones
Vasoconstrictor hormones include vasopressin, noradrenaline, adrenaline or angiotensin II.
Vasopressin in high doses causes vasoconstriction through the action on V1A receptor. It is used in intensive care.
Adrenaline acts through the α1-receptor as a potent vasoconstrictor. On the other hand if administered in low doses, its main action occur through the β2-receptor, thus acting as a vasodilatory agent in the blood vessels of the skeletal muscle, heart and the liver.
Noradrenaline posses high affinity to a α1-receptor (not to its β2 colleague), thus acting as vasoconstrictor agent in all tissues. Physiologically noradrenaline never reaches plasma concentration, that is high enough to overcome adrenaline β2-receptor vasodilator effect.
Intravenous administration of noradrenaline is used in intensive care unit to treat a severe hypotension.
Angiotensin II is a hormone with general vasoconstrictor effect. It is a part of renin-angioten-aldosterone system.
Vasodilator hormones
This group includes kinins, histamine, natriuretic peptides, VIP and partly adrenalin.
Bradykinin and kallidin are formed by the enzyme kallikrein, while plasma kininogens act as substrates. Through increase in NO production they induce dilatation of a vascular smooth muscle. Histamine also causes vasodilation through the action of NO. All three of substances also alternates the permeability of capillaries.
Natriuretic peptides (ANP – atrial natriuretic peptide and BNP – brain natriuretic peptide) are hormones synthesized in the atria and ventricles. Both have a significant vasodilator effect. They also increase natriuresis (by inhibition of sodium reabsorption in a distal tubule) and diuresis.
Neuronal regulatory mechanisms
Neural regulation of blood flow is mediated through sympathetic portion of autonomic nervous system. Noradrenaline is the main vasoactive substance, acting through α1-receptor. Sympathetic system is distinguished by a tone of a certain frequency which can be modified according to the needs of organism. Thus the degree of constriction depends on the current sympathetic tone – by decreasing the sympathetic tone vasodilatation is induced and vice versa.
An unique exceptions to the rule of sympathetic dominance are genital blood vessels and vasculature of sweat and salivary glands. Those are under the control of the parasympathetic innervation. Acetylcholine induce NO synthesis, thus acting as vasodilator agent.
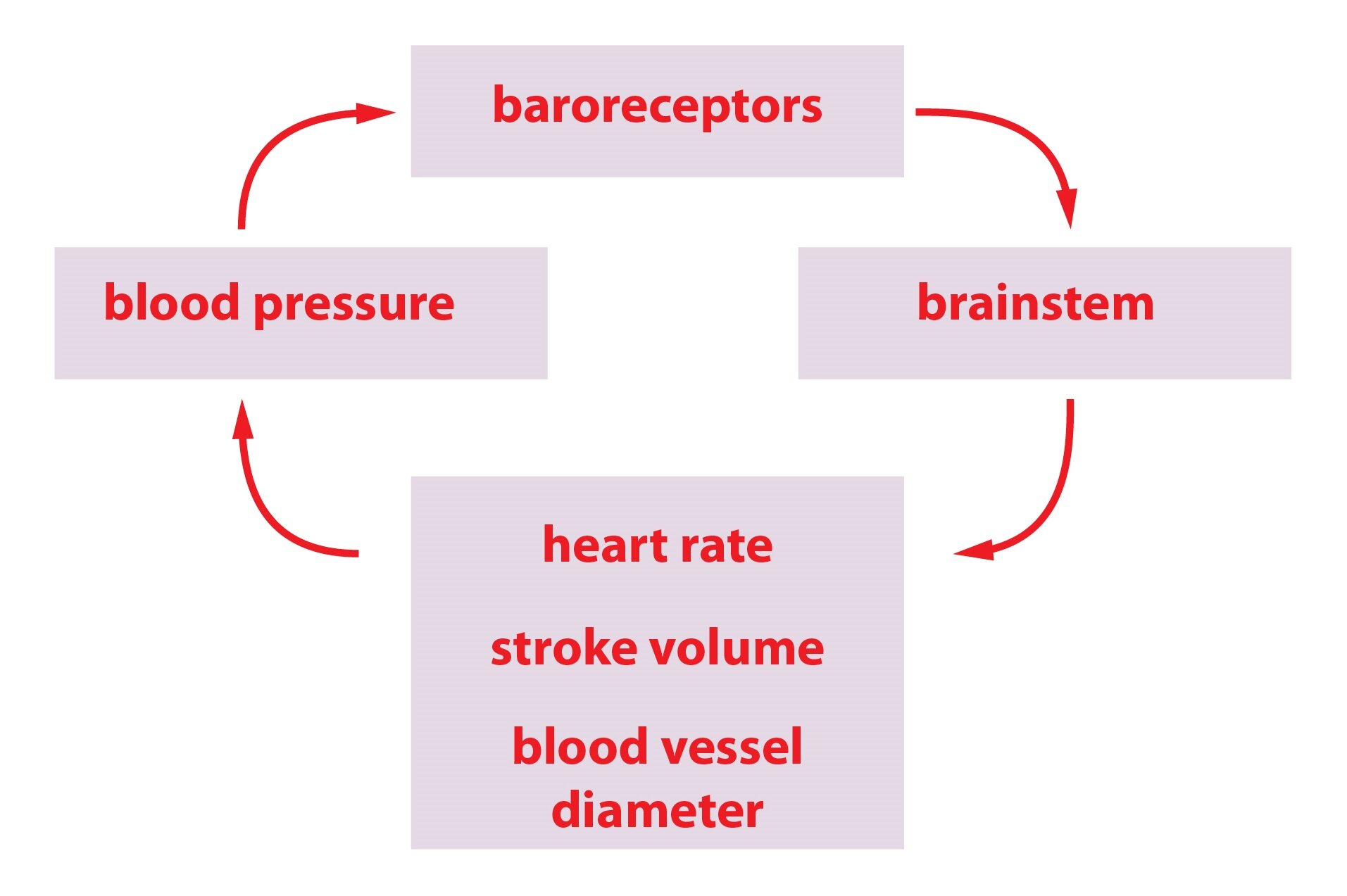
Vasoactive centre is located in the brain stem. It receive information directly from from the chemoreceptors and baroreceptors in the aorta and carotid arteries.
Chemoreceptors
Chemoreceptors are located in the carotid glomus and aortic arch. They have a high oxygen demand and are very sensitive to decrease in pO2. Changes in pH or pCO2 are also considered to be adequate modalities for stimulating the chemoreceptors. But if compared to the decrease in pO2, the chemoreceptor response is much weaker. If stimulated, chemoreceptors increase sympathetic tone, through the direct afferents into the vasoactive centre of the brain stem. Thus increasing the blood pressure.
On the other hand, central chemoreceptors of medulla and brain stem respond to changes in pCO2 and pH. pO2 is not likely to induce any response at all. Increase in pCO2 or decrease in pH greatly stimulates central chemoreceptors, which in turn increase sympathetic tone. Both cardiac output and peripheral vascular resistance increase.
Baroreceptors
Baroreceptors are mechanoreceptors, that are stimulated by a change in a blood pressure value. Receptor located in the carotid sinus is sensitive to a pressures around 180 mmHg. Receptor in the aortic arch reacts at even higher pressures. Activation of baroreceptors by high blood pressure value induces a decrease in sympathetic tone. Thus baroreceptor reflex is a mechanism involved in acute regulation of blood pressure.
Vasoactive centre constantly regulates the sympathetic tone, thus determining actual degree of peripheral resistance.
_
Microcirculation
The capillaries are designed for an exchange of nutrients, respiratory gasses and a fluid. High value of total surface area and their thin permeable walls contribute to their functionality. In fact capillaries are nothing more than a thin membrane composed of endothelial cells and their basal membranes. In some cases, capillaries posses pores with diameter smaller than the size of albumin is. There are several ways how the substances pass through the capillary wall – through interconnections between endothelial cells, pores, vesicle transport, diffusion or filtration. The main factor that determines the capillary blood flow is a value of pO2, which controls a tone of precapillary sphincter.
About 20 l of a fluid is filtered daily through the capillary wall. About 18 liters of filtered fluid is reabsorbed. The remainder is drained off through the lymphatic circulation. The main transport process in capillaries is a simple diffusion. Oxygen and carbon dioxide pass directly through the capillary wall, while ions, glucose and water pass through its pores.
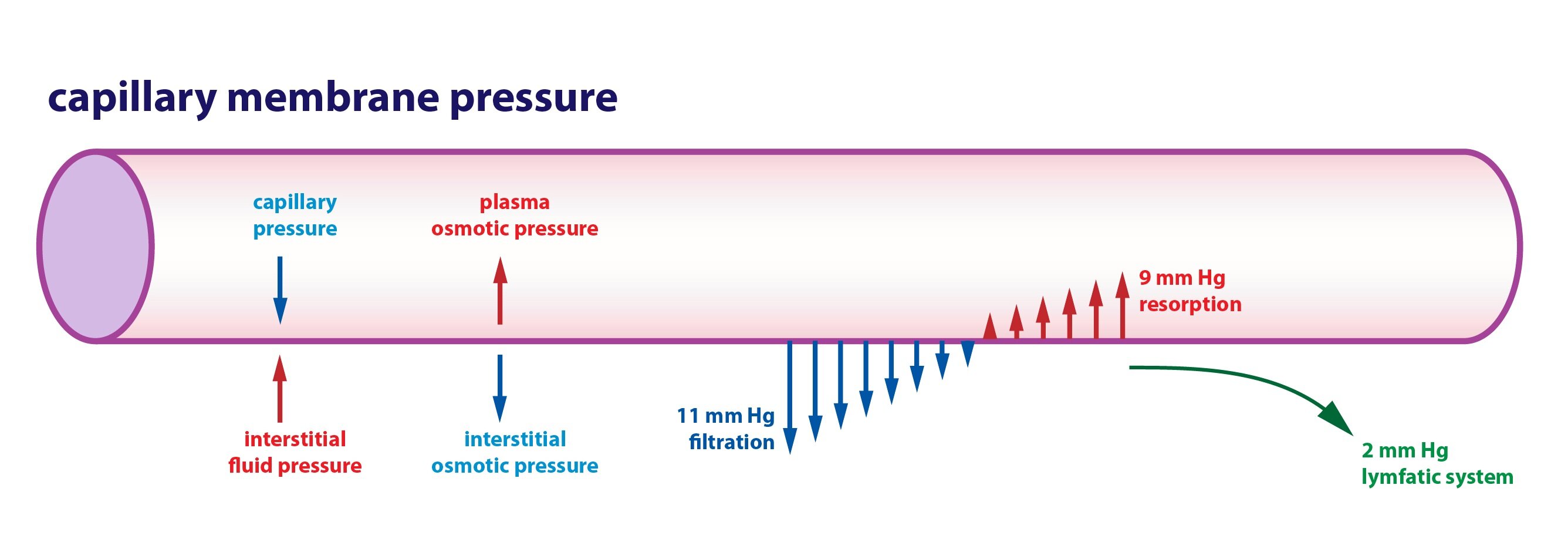
Movement across the capillary membrane is determined by Starling forces:
1) The capillary hydrostatic pressure – outwards from the capillary
2) Interstitial fluid pressure – its value depends on the particular organ – it is positive in the kidneys, liver and brain, negative in the skin
3) Capillary oncotic pressure – inside to the capillary
4) Interstitial oncotic pressure – outward from the capillary
Filtration rate differs according to the section of particular capillary. Main factor determining filtration rate is a difference of Starling forces.
1) Difference between capillary hydrostatic pressure and the interstitial hydrostatic pressure determines the hydrostatic pressure gradient.
2) Difference between capillary oncotic pressure and interstitial oncotic pressure determines the osmotic pressure gradient.
Filtration pressure exceeds oncotic pressure at the arterial end of capillary. In other words sum of the forces has an outward direction. Oncotic pressure exceeds the filtration pressure at the venous end of the capillary. In other words sum of the forces has an inward direction. Thus up to 90 % of the fluid is reabsorbed at the venous end of the capillary. Higher permeability of capillary wall at the venous end also contribute to the reabsorption.
Starling forces values may significantly differ from the general scheme in certain capillaries. For example, in kidneys fluid passes outward across the whole length of the capillary. The exact opposite is true in the gut.
_
Lymphatic circulation
About 20 l of a fluid is filtered daily through the capillary wall. About 90 % of the filtered fluid volume is reabsorbed. The remainder is drained off through the lymphatic vessels. Lymph is rich in proteins (including clotting factors), fats absorbed from the lymphatic system of the gut and immune cells. The flow of lymph is caused by a voluntary movement of skeletal muscles, valves of the lymphatic vessel, negative intrathoracic pressure during the inspiration and the suction effect of a bloodstream in the large veins. Pulsation of the smooth muscle in the walls of greater lymphatic ducts also contribute to some extent.
_
Venous return
Blood returns from organs to the heart through the venous system. Venous return is defined as the volume of a blood that returns to the right atrium in one minute. The venous return equals cardiac output under the physiological conditions,.
The venous return is determined by:
1) The residual pressure gradient – pressure difference between venules and right atrium. It acquires low values under the physiological conditions.
2) Negative heart pressure – negative pressure originating in the right atrium during the diastole – the suction effect of the heart.
3) Muscle pump – a contraction of skeletal muscles compress the veins.
4) Intrathoracic negative pressure – inhalation further decreases the negative pressure in the thoracic cavity. This leads to dilatation of blood vessels in the chest. Resulting in suction of the blood into the large veins.
5) Positive pressure in the abdominal cavity during inspiration – due to contraction of diaphragm.
6) Venous valves assure one way flow of blood.
Blood vessels in the lower extremities are affected by an increased hydrostatic pressure of the blood column, while an individual stands. Increase in hydrostatic pressure leads to a dilatation of the blood vessels. Thus the circulating blood volume reduces by about 0.4 l. Decrease in circulating blood volume leads to a decrease in venous return and cardiac output. Pathological phenomenon called an orthostatic collapse may occur. This can be prevented by an activation of orthostatic reflex. Low pressure stimulates baroreceptors, which in turn increase sympathetic tone. Increased sympathetic activity raises cardiac output and causes vasoconstriction, thus normalize a blood pressure value.
Central venous pressure (CVP) is a mean pressure in the superior vena cava (which is identical with the pressure in the right atrium). It reflects the pressure in the right ventricle at the end of diastole. CVP depends primarily on the volume of blood and its normal value is about 0-9 mmHg. The increase in CVP may indicate heart failure.
_
Fetal circulation
Correct function of the placenta is essential for the fetus. Placenta provides fetal tissues with oxygen and nutrients as well as provide disposal of metabolites and CO2 .
Oxygenated blood from the placenta flows through the umbilical veins. It passes through venous duct to the inferior vena cava. Then blood continue to the right heart, passes through opened oval foramen to left heart and then to the aorta. Blood from the upper half of the body partially mixes with blood from the lower half of the body in the right atrium. The blood from the upper half of the body flows from the superior vena cava through the right atrium to the right ventricle and then to the pulmonary artery. From pulmonary artery it flows through the arterial duct into the aorta. Arterial duct merges with aorta past the great aortic branches for the head and upper extremities. Thus the brain and the arms are supplied by oxygen rich blood. The mixed blood then enters umbilical arteries as well as lower half of a body.
After birth, umbilical vessels are interrupted. Level of pCO2 rises and stimulates first inspiratory movement. Inspiratory movement causes a negative pressure in the chest cavity, leading to a suction of the blood from the umbilical vein and the placenta. With the inflation of the lungs, the pulmonary circulation resistance significantly decreases. This changes the direction of blood flow in the arterial duct. Blood flows into the vasculature of the lungs causing sudden increase in left atrial pressure, therefore closing the oval foramen.
The arterial duct, venous duct and oval foramen obliterates after the birth (up to two weeks).
Subchapter Authors: Peter Ivák, Josef Kroupa, Patrik Maďa and Josef Fontana