Content:
1. Functional morphology of liver
2. Function of liver
3. Laboratory liver function tests
4. Biotransformation of xenobiotics
_
Functional morphology of liver
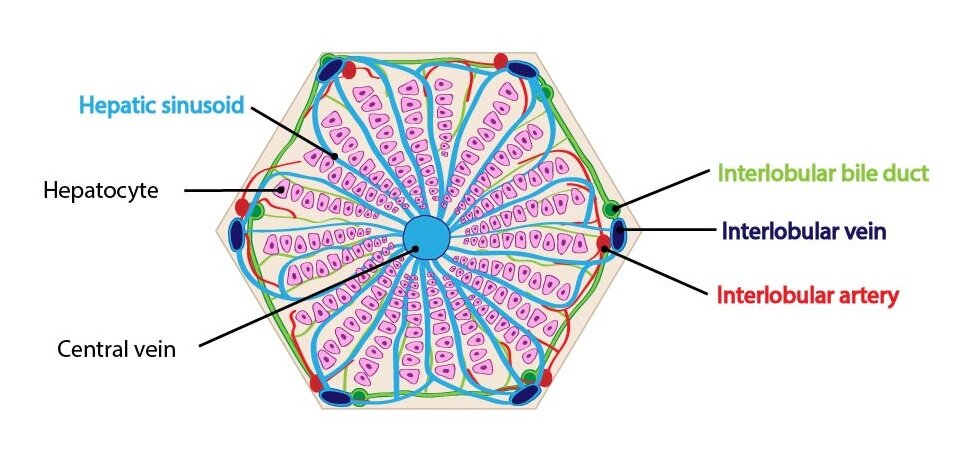
Liver is the second largest organ in the body (the largest is skin), weighing approximately 1,5 kg. Liver composes of four lobes divided into lobules which are surrounded by blood vessels. Branches of the portal vein and hepatic artery enters the lobes and transport oxygenated blood and nutrients into sinusoids (sinusoidal capillaries) surrounded by hepatocytes. Liver develops as an endodermal hepatic diverticulum of foregut with mesodermal component of septum transversum. The liver is surrounded by fibrous connective tissue capsule – capsule of Glisson.
Liver parenchyma consists of hepatic epithelial cells – hepatocytes, arranged in the cellular cords. On the section, these cords remind stripes of cells. In an adult, each cord is formed by one line of cells. In children (approximately until 6 years) is each cord formed by two lines of cells. Between these cellular cords are located venous sinuses. These sinusoids are lined by fenestrated discontinuous endothelium which lacks basal lamina. Important components of hepatic sinusoids are Kupffer cells located on the luminal surface of endothelial cells. Kupffer cells belong to the mononuclear phagocytic system (typical macrophages). The space between basal surface of endothelium and plates of hepatocytes is called the perisinusoidal space (space of Disse). In this space, the exchange of substances between liver and blood is located. The hepatic stellate cells (Ito cell) are found in perisinusoidal space. These cells store lipid droplets and vitamin A in their cytoplasm. In a pathologic conditions, hepatic stellate cells differentiate into myofibroblasts and synthesize collagen fibers into the space of Disse. This fibrous tissue replaces the hepatocytes, possibly resulting in liver fibrosis which can progress into hepatic cirrhosis.
.In the liver, these cells are found: the hepatic stellate cells (Ito cells), sinusoidal endothelial cells, macrophages (Kupffer cells), epithelium of bile canaliculi (from simple cuboidal to simple columnar), fibrocytes in the connective tissue capsule, trabeculae and interlobular septa, hepatocytes.
Hepatocytes
The hepatocytes are large cells with central nuclei. Represent 80 % of the cell population of liver. The hepatocyte cytoplasm contains numerous mitochondria, rough and smooth endoplasmic reticulum, deposits of glycogen, lipid droplets, large number of lysosomes and peroxisomes and multiple Golgi complexes. The both free sides of hepatocyte have prominent microvilli. Between two surfaces of neighboring hepatocytes, the bile canaliculi are located. Bile canaliculi are the smallest bile canals and their walls are created by surfaces of adjacent hepatocytes. The bile flow direction is opposite to the blood flow (from the centre toward the periphery). The terminal parts of bile canaliculi are called canals of Hering. The canals of Hering already have epithelial lining (simple flattened or cuboidal cells called cholangiocytes) and they empty into the interlobular bile ductules.
Functional organization of the liver parenchyma
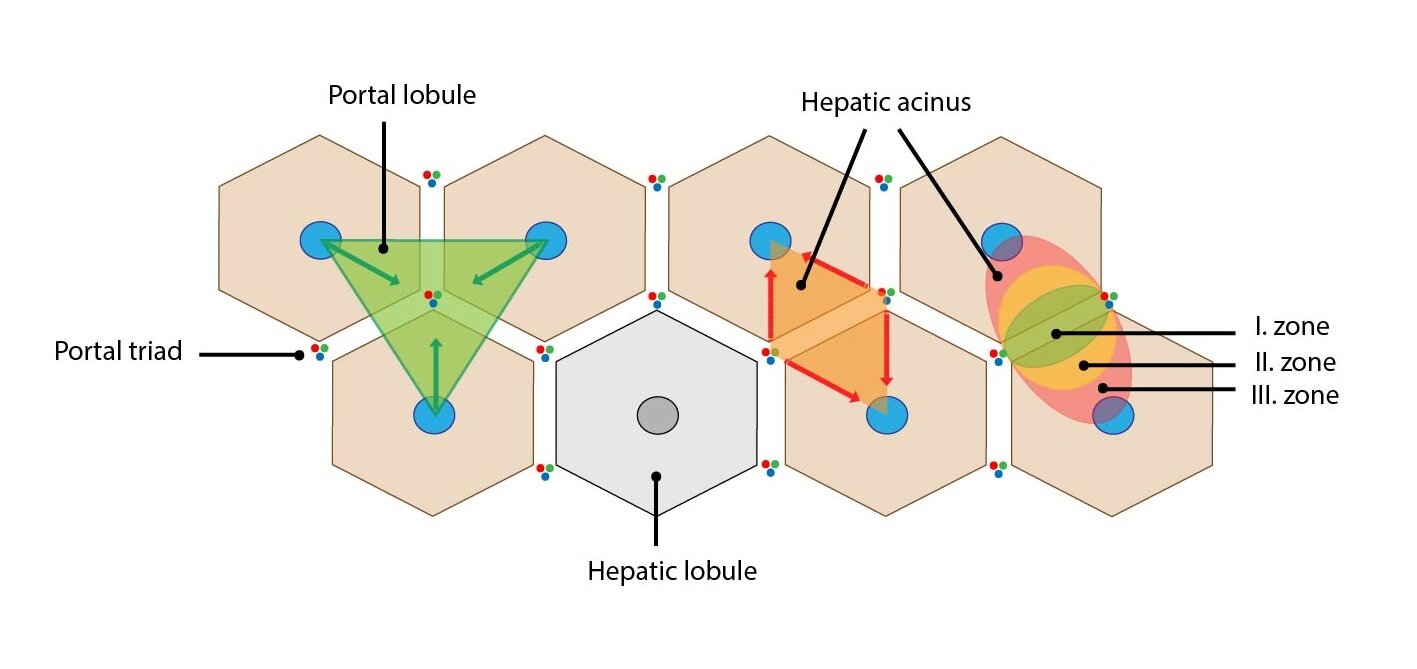
There is several options how to describe structural organization of liver and thus understand its function. Specifically, we can use three structural and functional unites – liver lobule, portal lobule and liver acinus.
Liver lobule
Liver lobule is a classic structural unite describing the organization of liver parenchyme. It is based on the distribution of branches of the portal vein and hepatic artery within the liver. It is composed by polygonal-shaped parenchyma bordered by thin layer of collagenous connective tissue. In this connective tissue layer, the portal triad is localized. The portal triads composed of the branches of portal vein, hepatic artery and bile duct. One liver lobule contains 3 to 6 portal triads.
The liver lobule itself is composed of anastomosing plates of hepatocytes separated by hepatic sinusoids. Hepatocytes are oriented radially to periphery of lobule. In the centre of lobule is central vein which leads blood into the hepatic veins.
Portal lobule
The portal lobule (lobulus venae interlobularis) is a triangular block of liver parenchyma supplied by circumlobular branches of one interlobular vein – at the centre is portal triad and at the peripheral angles are located three central veins. This lobule represents the portion of hepatic parenchyma that secrete bile that drains into the bile duct of portal triad in the centre of portal lobule.
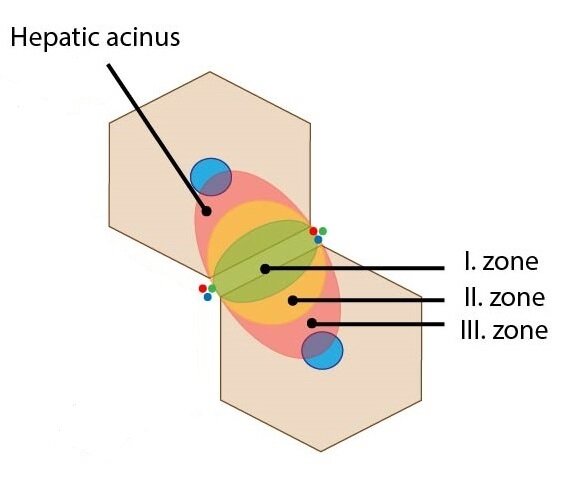
Liver (portal) acinus
The liver acinus is functional unit of liver parenchyma providing the best correlation between blood perfusion, metabolic activity and pathological processes in liver. This ellipse-shaped structure is the smallest functional unit of liver parenchyma. The short axis of acinus is defined by connecting line between adjacent portal triads and the long axis is defined by connecting line between two adjacent central veins. It remotely resembles two triangles attached together by their bases with central veins in vertices. Between their bases are located the circumlobular vein and artery which supply sinusoids of the liver acinus.
Depending on distance from the circumlobular vein and artery (thus depending on distribution of oxygenated blood and nutrients), three distribution zones are defined. These elliptical zones surround the short axis of acinus:
1) Zone I – represents the portion of parenchyma closest to the circumlobular vein and artery – it is the first to receive oxygen and nutrients. The oxidative metabolism predominates here (e.g. gluconeogenesis and proteosynthesis).
2) Zone II – lies between zone I and zone II – receives less oxygenated blood
3) Zone III – lies farthest from circumlobular vein and artery (closest to the central veins) – the least oxygen gradient. The reduction processes are predominant in this location – e.g. detoxication.
This classification is also important for description of pathological conditions – selective damage of hepatocytes.
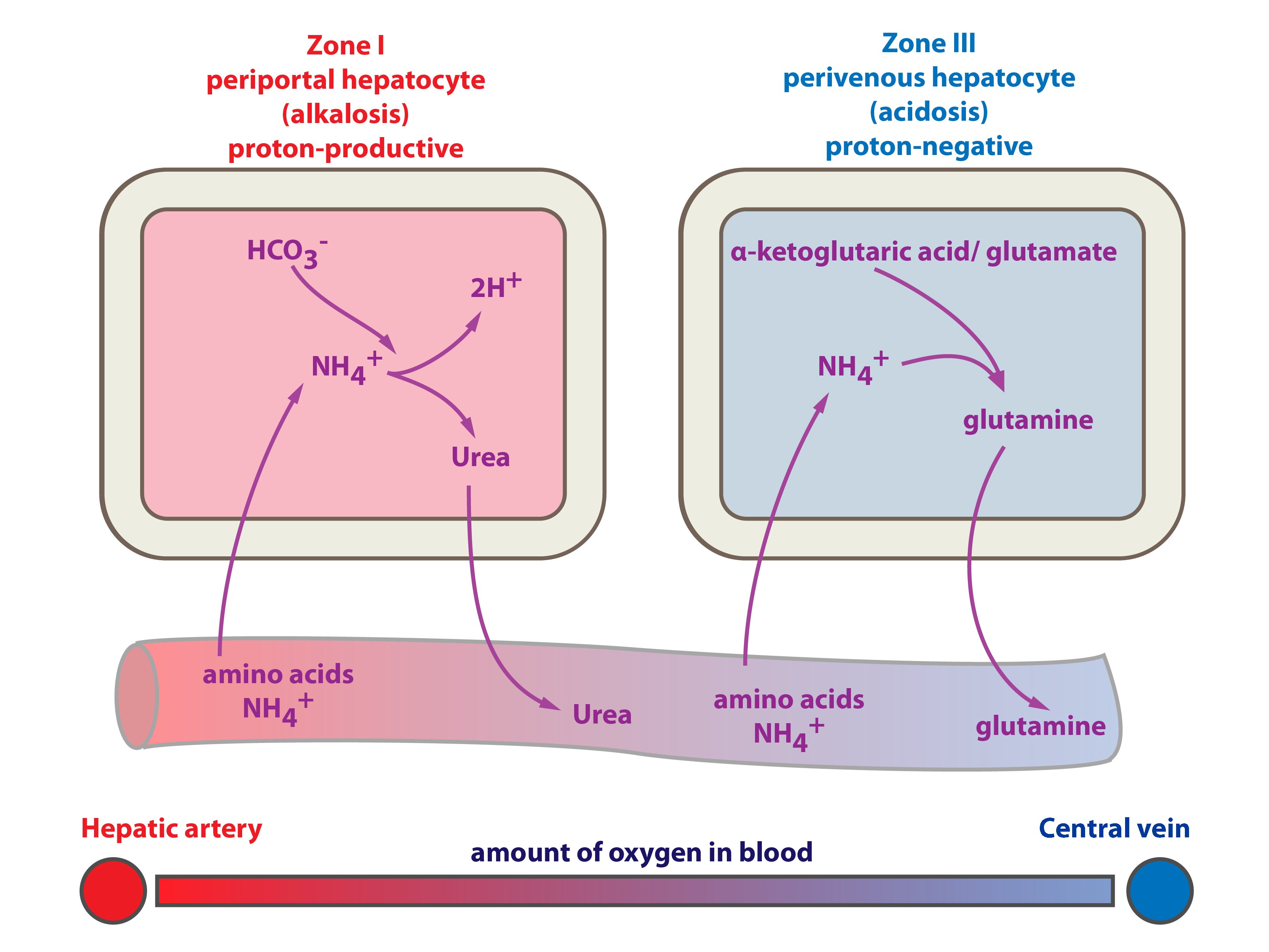
On the basis of described classification, the hepatocytes can be distinguished into periportal and perivenous hepatocytes.
Periportal hepatocytes are the first to receive blood from portal vein and hepatic artery. The oxygen (↑ pO2) and nutritive supply is the highest and the cells contain more mitochondria and less sER. That is the reason why oxidative metabolism predominate here (e.g. Krebs cycle, oxidative phosphorylation, β-oxidation, gluconeogenesis, proteosynthesis). The ammonia detoxification is here represented by synthesis of urea.
Perivenous hepatocytes are supplied by poorly oxygenated blood (lower pO2) with less nutritions. The reduction processes are predominant here (typically glycolysis, lipid synthesis, biotransformation of xenobiotics on sER). The ammonia detoxification is here represented by glutamine synthesis.
_
Liver function
Hepatocytes have very exceptional post in intermediary metabolism. It has important role in (1) maintenance of internal environment (i.e. homeostasis), (2) reciprocal conversions of nutrients, (3) regulation of storing and releasing energy, (4) modifications of hormones and vitamins, or (5) in harmful compounds detoxification (xenobiotic biotransformation). The liver has also secretory function – it produces bile. The bile is necessary for lipid digestion. It has also another function, it excretes a lot of compounds from the body. The liver has endocrine function (it produces (1) erythropoietin (only 10 %; remaining 90 % is produced in the kidneys), (2) calcidiol, (3) insulin-like growth factors – somatomedins). The liver has role in immune processes (e.g. acute phase reactants synthesis, Kupffer cells). Now you can easily deduce that the liver is absolutely necessary for life, therefore severe liver failure leads to death.
Before single functions will be described one important question has to be answered. What are the sources of energy for the liver?
This is important because of fact that many functions of the liver are energetically demanding. Hepatocytes predominantly use aerobic phosphorylation, hence the liver consume 20-30 % of all oxygen, 90 % of it is used for macroergic phosphates synthesis. In normal conditions β-oxidation and amino acids degradation are the main sources of energy.
Saccharide metabolism
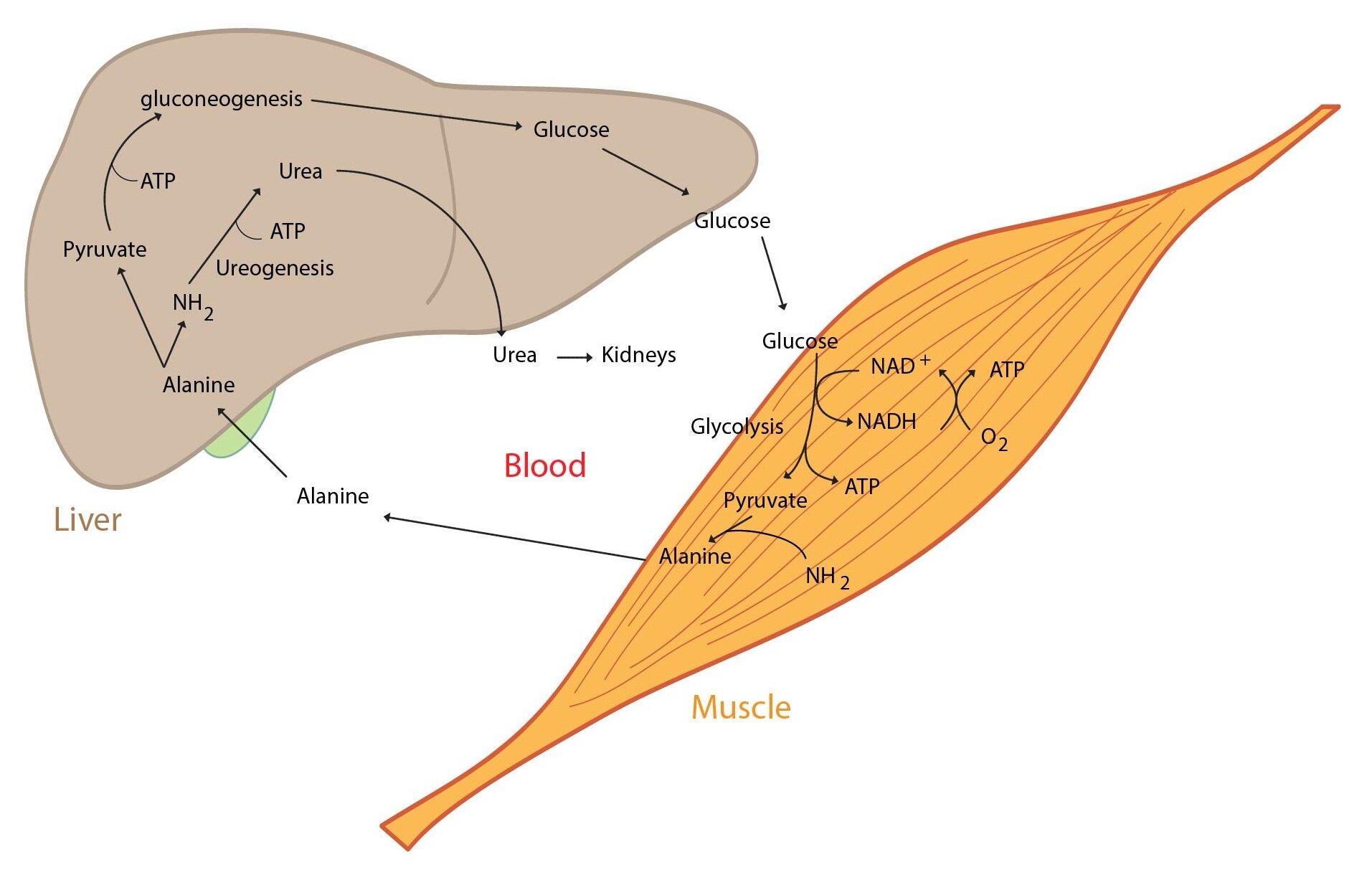
It is vital for the body to maintain blood glucose within physiological range (i.e. 3,3 – 5,6 mmol/l). The liver have so called glucostatic function, it include (1) short-term regulation (hours) and (2) long-term (days, weeks) regulation of glycemia. There are many metabolic pathways that are turned on or off according to glycemia. This changes in activity are caused by hormones (produced by endocrine pancreas or adrenal glands). Liver glycogen is buffer of glycemia. There is one key fact: hepatocytes contain glucose-6-phosphatase (cleaves off phosphate from Glc-6-P). This enzyme allows the liver to release free glucose to the blood stream. The liver is part of two important interorgan pathways – the Cori cycle and glucose-alanine cycle.
Now we describe some metabolic conditions and manner of the liver reaction:
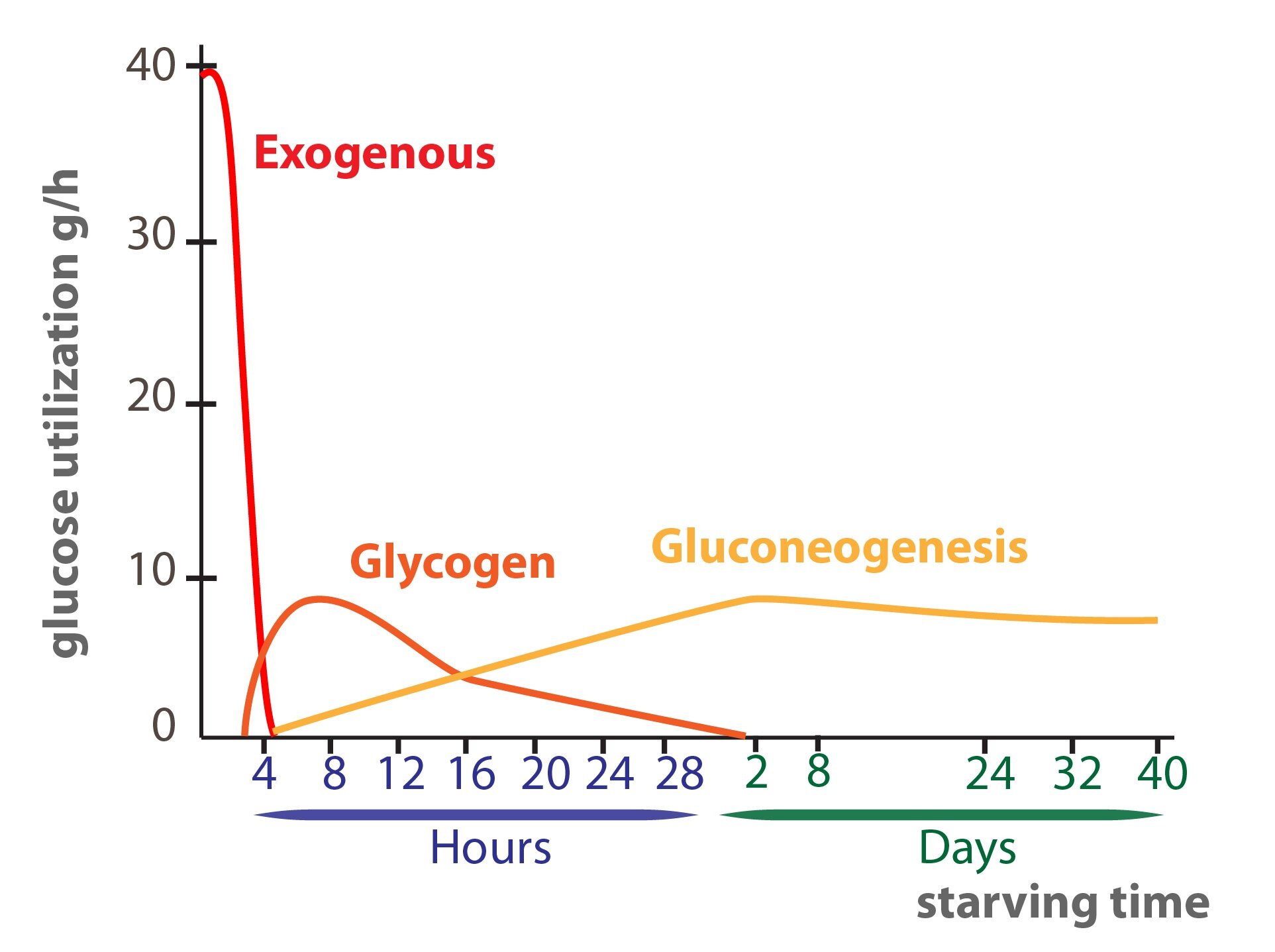
1) High glucose concentration in the portal blood after a meal. This leads to glycogen synthesis in the liver – hepatocytes absorb glucose and produce glycogen. In high glucose excess (and full liver glycogen stores) is glucose converted to fatty acids and then to TAG.
2) In fasting glycemia decreases this in turn leads to glycogen decomposition (glycogenolysis) and then is glucose released to the blood.
3) In prolonged fasting glycogen stores become depleted and the liver starts to produce glucose from non-saccharide substrates (lac, glucogenic amino acids, glycerol) – gluconeogenesis.
Cytoplasmic membrane of hepatocyte is well permeable for glucose (insulin is not necessary). After entry of glucose to hepatocyte it is phosphorylated to glucose-6-P by the enzyme glucokinase (KM ~ 10 mmol/l, it is independent on Glc-6-P concentration). Glc-6-P then enter some of its metabolic pathways. Apart from mentioned above Glc-6-P may be used for saccharide derivatives synthesis (amino saccharides, uronic acids) or in the pentose pathway.
In the liver also take place (1) conversion of other saccharides to glucose, (2) fructose metabolism, (3) galactose metabolism, (4) amino saccharides synthesis, (5) uronic acids synthesis, and (6) energetic metabolism regulating hormones degradation (e.g. insulin, glucagon).
Lipid metabolism
The liver is central organ for lipid metabolism. Some metabolic pathways of lipids are unique for the liver (e.g. ketone bodies synthesis. Many pathways take place in other tissues, in the liver these are however quantitatively the most important. Main examples of the lipid metabolism are as follows:
1) Fatty acids oxidation and ketone bodies synthesis
Hepatocytes are extremely active in fatty acids oxidation for energy production. In starving the liver oxidize much more fatty acids than it would need for its energy demands. The liver synthesizes ketone bodies (ketogenesis), ketone bodies are released to the circulation for other tissues (ketone bodies are utilised only in extrahepatic tissues). The liver in the same time use also second compound of TAG – glycerol. Glycerol is substrate for glucose production – gluconeogenesis.
2) Fatty acid and TAG production
There is very intensive fatty acids and TAG synthesis in hepatocytes. The liver is the main location of glucose conversion to pyruvate. PDH then produces AcCoA and fatty acids and TAG are thus synthesized. Glucose used for this conversion comes from excessive saccharide income. TAG are exported by VLDL and stored in the adipose tissue. Part of fatty acids is used for phospholipid synthesis.
3) Lipoprotein metabolism
Hepatocytes have key role in lipoprotein metabolism. (1) VLDL synthesis, (2) partially HDL synthesis, (3) conversion of IDL to LDL, (4) chylomicron remnants, HDL, and LDL degradation. All of these take place in the liver.
4) Metabolism and excretion of cholesterol and phospholipids
Hepatocytes are important location of cholesterol and phospholipids synthesis. Part become a part of lipoproteins and thus it is transported to the extrahepatic tissues, part is excreted to the bile (cholesterol also as the bile acids).
In smooth endoplasmic reticulum of hepatocytes cholesterol excess is converted to bile acids by hydroxylases that use O2, NADPH, cytochrome P450. More details below.
Protein and amino acids metabolism
The most important processes in protein and amino acids metabolism that take place in the liver are as follows:
1) Amino acids metabolism
a) Amino acids deamination and transamination with subsequent conversion of carbon skeleton to glucose or lipids
The liver controls amino acids levels in plasma. Amino acids come to the liver as products of (1) digestion of ingested proteins or (2) degradation of body proteins. Amino acids undergo specific reactions in hepatocytes – e.g. deamination (cleavage of amino group), decarboxylation (cleavage of –COOH group), or transamination.
Very representative example of transamination is reaction of alanine with α-ketoglutarate yielding pyruvate and glutamate. This reaction is catalysed by the enzyme alanine aminotransferase (ALT). Next transamination reaction is reaction of aspartate with α-ketoglutarate yielding oxaloacetate and glutamate, catalysed by the enzyme aspartate aminotransferase (AST). Both ALT, and AST are used as diagnostic markers of liver cells damage.
Single amino acids are degraded by unique catabolic pathways, their carbon skeletons are however converted to one of these seven compounds: pyruvate, acetyl-CoA, acetoacetyl-CoA, α-ketoglutarate, succinyl-CoA, fumarate, and oxaloacetate that may be converted to saccharides or lipids.
b) Non-essential amino acids synthesis
In the liver is produced a lot of non-essential amino acids.
2) Ammonium detoxification and its elimination from the body
In the amino acids metabolism certain amounts of ammonium are produced. Ammonium is toxic (particularly for central nervous system). Its main source is amino acids deamination, part of ammonium is derived from glutamine hydrolysis to ammonium and glutamate. Ammonium detoxification is provided by (1) urea synthesis (the ornithine cycle) or (2) glutamine synthesis.
3) Synthesis of majority of plasma proteins (apart from immunoglobulin)
As an example we use: albumin (hypoalbuminemia causes edemas) or coagulation factors (liver cirrhosis may lead to life threatening bleeding).
Liver metabolism of other nitrogen compounds
Except proteins and amino acids in the liver however take place metabolism of many other nitrogen compounds:
1) Purine degradation – uric acid production
2) Creatine and choline synthesis
3) Heme catabolism
3) Heme catabolism
In normal conditions majority of erythrocytes is destroyed in the reticuloendothelial system (RES) of spleen, bone marrow, and liver (part of erythrocytes is disintegrated in hypodermis or in the blood stream).
Fate of single haemoglobin compounds is as follows:
a) Globin → hydrolysis to single amino acids → their metabolism
b) Heme → biliverdin → bilirubin → bile pigments
c) Released Fe3+ cation is transported in the blood bound to the protein transferrin and it is used again
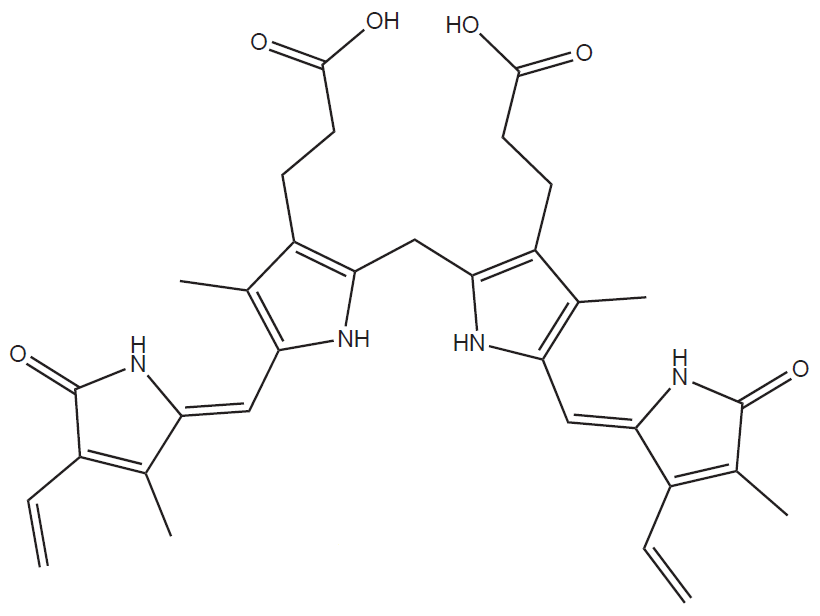
Heme is cleaved and oxidised to green pigment biliverdin (linear tetrapyrrole). This reaction is catalysed by the enzyme heme oxygenase that is localised in endoplasmic reticulum:
Heme + 3 NADPH + 3 O2 → biliverdin + Fe3+ + CO + 3 NADP+ + 3 H2O
Then reduction of biliverdin to bilirubin (orange-red colour) takes place catalysed by the enzyme biliverdin reductase.
Bilirubin is insoluble in water, therefore it has to be bound to albumin in the blood (this is so called unconjugated = indirect bilirubin). In the liver is bilirubin conjugated with glucuronic acid. This reaction is catalysed by the enzyme UDP-glucuronyltransferase. The reaction produced bilirubin monoglucuronide or bilirubin diglucuronide that is water soluble. Therefore it is possible to excrete it to the bile and thus to the intestine.
In the intestine deconjugation takes place and then bacteria cause reduction of bilirubin to urobilinogen and stercobilinogen. Part of urobilinogen is resorbed from the intestine back to the blood and it is transported to the urine. Uro- and stercobilinogen are converted by bacteria to stercobilin and urobilin. These are eliminated in the stools. More detail: viz Subchapter 6/4.
Production, composition and function of the bile
The bile is thick, bitter, yellow-green liquid produced by hepatocytes. Its daily production is approximately 0,5 – 1 litre and bile pH is within 6,5 – 8. The bile is transported from the liver to the gallbladder, there its thickening takes place
Bile composition
97 % of bile is water. Bile also contains bile acids, inorganic ions (Na+, Cl–, HCO3–), cholesterol, phospholipids, bile pigments etc.) Bile on contrary does not contain enzymes (apart from liver isoenzymes of alkaline phosphatase). Cholesterol solubility is improved by present phospholipids and bile acids – together they form micelles.
Bile produced by the liver is significantly thickened in the gallbladder (dry basis increases to 14 %). Organic compounds become concentrated.
Bile function
Although bile does not contain digestive enzymes, it is crucial in digestion and absorption of lipids (more details…) Second important function is its excretion function – many metabolites, foreign substances (mainly with Mr > 300) and cholesterol are excreted from body by bile.
Bile acids
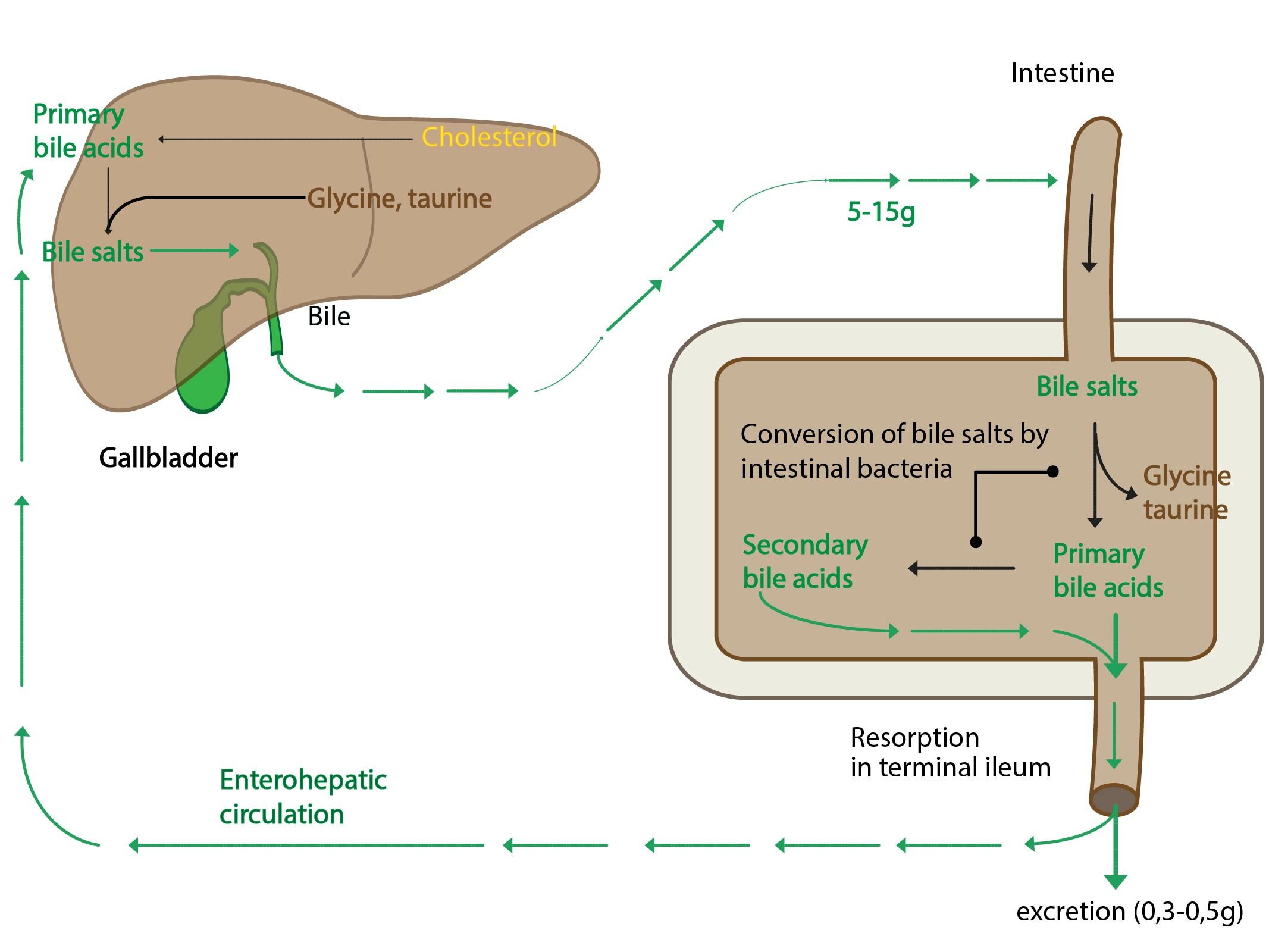
Bile acids are produced in the liver from cholesterol and they serve as its degradation product (daily production is approximately 350 mg bile acids). All bile acids have 24 carbon atoms and complete steran core (human body is not capable of decomposition of steran core). In the bile they are present as salts with Na+ or K+ in addition they are conjugated with glycine or taurine (conjugation also takes place in the liver). Taurine is abundant intracellular amino acid that is produced in cysteine metabolism. Its precise function is unknown, probably it defends body against toxic intermediates.
Bile acids are divided as follows:
1) Primary bile acids – cholic acid and chenodeoxycholic acid
2) Secondary bile acids that are produced from primary bile acids by intestinal bacteria. Secondary bile acids are deoxycholic acid and lithocholic acid.
Every day is excreted 5-15 g of bile acids. You should recall that daily production is only 350 mg. This is allowed by enterohepatic circulation – approximately 95% of bile acids salts is reabsorbed in the small intestine (in terminal ileum – simple diffusion or sodium cotransport) and transported in the portal blood back to the liver. Only few is lost in stools. This eliminates excessive body cholesterol.
Bile acids cause lipid emulsification in lipid digestion and absorption in the intestine. Bile acids with cholesterol and phospholipids are parts of mixed micelles. Bile acids are important for intestinal absorption of lipophilic vitamins.
Clinical correlation:
Bile stones (concrements) are made up of normal compounds of bile (cholesterol and bile pigments).
Role of the liver in vitamin metabolism
Liver has important role in metabolism of vitamins. In the liver (1) takes place conversion of provitamins to vitamins (e.g. carotene → vitamin A), (2) vitamins are stored (lipophilic vitamins, vitamin B12, (3) conversion to active forms of vitamin (25-hydroxylation of vitamin D → calcidiol, production of enzyme cofactors – mainly B-vitamins)
Liver and the trace elements metabolism
Liver is important in metabolism of many trace elements.
1) Transport protein synthesis – transferrin, ceruloplasmin
2) Storage of trace elements: Fe (ferritin) and other (Cu, Mn, Co, Mo, Zn,…)
3) Deiodination of thyroid hormones
_
Laboratory liver function tests
There are two liver-specific enzymes assessed in basic set of biochemical examinations – alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Apart from these two enzymes more parameters are used by doctors to determine liver function status – e.g. γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), bilirubin levels etc.
Alanine aminotransferase is enzyme localised predominantly in cytoplasm of hepatocyte. Therefore ALT gets to plasma even in mild damage of hepatocyte membrane (hence ALT catalytic activity in plasma increases). The enzyme aspartate aminotransferase is contrariwise localised mainly in the hepatocyte mitochondria. Increased activity of AST in plasma indicates more severe damage of hepatocytes. Catalytic activities of both ALT and AST are manifold increased in liver diseases – e.g. acute viral hepatitis or acute toxic damage. Mild increase of ALT, AST activities has been seen in alcohol overdose. ALT, AST activities are also increased in liver cirrhosis.
γ-glutamyltransferase is also assessed in liver status examination. GGT catalyses transfer of γ-glutamyl from glutathione to amino acids thus allowing them to pass cell membrane. GGT is the most sensitive marker of alcohol liver damage (i.e. GGT is increased in alcoholics). However GGT is also increased in cholestasis (that is bile ducts obstruction).
Alkaline phosphatase (ALP) includes several isoenzymes. Increased activity of the liver isoenzyme ALP are marker for example of bile ducts obstruction, hepatitides, cirrhosis, liver metastases.
Bilirubin levels assessment is also of great importance. Total bilirubin, direct bilirubin and indirect bilirubin in serum are thus determined. Direct bilirubin is alternative name for conjugated bilirubin (i.e. bilirubin + 2x glucuronic acid). Increased non-conjugated (i.e. indirect) bilirubin is typical in haemolytic anaemias, newborn jaundice and inherited defect of bilirubin conjugation. Increased conjugated bilirubin is typical in obstruction of bile flow to gut. Total bilirubin is manifold increased in viral hepatitis.
Physiologic values in serum:
Total bilirubin: up to 22 µmol/l
Conjugated (direct) bilirubin: up to 5,1 µmol/l
ALT up to 0.75 µcat/l
AST up to 0.75 µcat/l
ALP up to 2.29 µcat/l
GGT men 0.25 – 1.77 µcat/l, women 0.17 – 1.10 µcat/l
_
Biotransformation of xenobiotics
A xenobiotic is compound foreign for the organism. It may be of natural origin or it may be produced by human. Therefore even pharmaceuticals are xenobiotics.
Xenobiotics are rarely eliminated from the body unchanged. Majority of them undergo in the body conversion of various extent. Higher animals have developed highly efficient mechanisms for detoxification and elimination of xenobiotics.
The aim of biotransformation is conversion of xenobiotic to ineffective metabolite that is easy to eliminate. In the other words detoxification and increased polarity ease excretion. In some compounds however their toxicity is increased after biotransformation. Biotransformation processes are influenced by many factors: age, sex, genetics, diseases, etc.
Xenobiotic biotransformation takes place predominantly in smooth endoplasmic reticulum, but also in mitochondria, lysosomes and cytosol. Used enzymes perform at the same time metabolism of endogenous compounds. Xenobiotic metabolism typically occurs in two phases: biotransformation itself and conjugation. However it is not true that all xenobiotics must undergo both phases, i.e. some substances enter directly second phase, some substances may be excreted without conjugation.
Entry of xenobiotic to the body
Xenobiotics enter the body by several different paths. Mainly it is through alimentary tract (peroral entry), skin (dermal entry), respiratory system (inhalant entry), or injection administration (intravenous, intramuscular or subcutaneous entry).
In the alimentary system the majority of substances is absorbed either in stomach, or in intestines. From these organs they are transported either in portal vein to the liver, and then to systemic circulation (polar xenobiotics), or in lymphatic system directly to systemic circulation (lipophilic compounds). Some substances are able to be absorbed in mouth (sublingual drug administration).
Substances entering through every other path than the alimentary tract get to the liver later.
Xenobiotics biotransformation of course is not localised only in the liver. It takes place intensively in the spots of entry (the lungs, alimentary tract, skin…) and places of elimination (the kidneys, GIT, etc.)
Chemical features of xenobiotic
There are two basic groups of xenobiotics according to their chemical feature (polarity) – polar and nonpolar.
Polar (hydrophilic) xenobiotics are well soluble in water. They get through membranes poorly – they must use channels and transporters. In the blood stream they are transported freely (transport protein is not needed) and they are rapidly eliminated in the urine.
Nonpolar (lipophilic, hydrophobic) xenobiotics are water insoluble or poorly soluble. They get through membranes freely or they may get stuck in membranes. In the blood stream transport protein is needed – thus they are slowly eliminated (since bound to proteins they cannot reach the urine). Aim of lipophilic xenobiotics metabolism is to change their molecule to make their elimination easier.
Distribution (or partition) coefficient (P) determines the ability of the compound to pass biological membrane:
P = c(L)/c(W)
c(L) is concentration of the compound in the lipid phase (n-octanol) and c(W) is concentration of the compound in the water phase. This coefficient gives ratio of distribution of xenobiotic between lipid bilayer of biomembrane and water. The higher is ratio (i.e. the compound is more soluble in lipids), the easier xenobiotic passes biomembranes using simple diffusion.
Transport protein binding
Transport protein binding is not covalent. Xenobiotic is rather bound by ion and hydrophobic interactions. This binding depends on mutual affinity, presence of other transported substances (that thus compete of binding) or on concentration of the compound or of the transport protein. Certain portion is always free (it is biologically active portion). Bound portion is “store form” in the blood stream (the binding is reversible, thus when free portion is decreased, store portion provides more substance – it dissociates from the transport protein). Only free portion is able to enter hepatocyte or be filtered in the kidneys. Therefore transport protein binding slows elimination of xenobiotic from the body.
Xenobiotic fate
Fate of single xenobiotics may be quite different.
Metabolisable (degradable) substances are used in metabolism – e.g. ethanol is oxidised to CO2 and water.
Substances poorly soluble in water are converted to more soluble metabolites (below in this text – biotransformation and conjugation). This eases their elimination from the body. Excretion takes place in the urine (smaller substances – glucuronides with Mr < 300) or in the faeces (by eliminating to the bile) (bigger substances – glucuronides with Mr > 300).
In metabolism of some substances toxic by-products (e.g. reactive oxygen species) or biologically active intermediates/products are produced. These are capable of serious damage to the body. The original molecule of the xenobiotic may be almost or entirely harmless. However the same process is sometimes desirable – some compounds are administered in form of biologically inactive precursors, and their activation takes place in the body by biotransformation.
First (biotransformation) phase – biotransformation itself
In the first phase new functional groups are inserted to the molecule of xenobiotic. This has two goals: (1) increase polarity of xenobiotic, (2) produce places in the molecule that are capable of conjugation. These changes take place predominantly in smooth endoplasmic reticulum. First phase reactions may be very various. Examples are as follows: hydrolytic cleavage (e.g. esterases, peptidases), oxidation, reduction, alkylation, dealkylation (O- or N-), desulfurization of thiol compounds (change of S for O) or methylation (O- or N-).
Monooxygenase system – MFO (mixed function oxygenase)
The most common manner of biotransformation is oxidation of xenobiotic. Oxidation (or rather oxygenation) reactions are catalysed by enzymes oxygenases – the most important are so called mixed-function oxygenases. This system may be found on cytosolic side of endoplasmic reticulum and consists of three components:
1) Flavoprotein – NADPH cytochrome P450 reductase
2) Hemoprotein – cytochrome P450
3) Lipid component – phosphatidylcholine
Because of presence of cytochrome P450 this system is also called enzyme system of cytochrome P450 – CYP.
MFO have both oxidase and oxygenase ability, i.e. in reaction the substrate is oxygenated and NADPH is oxidised. Reductive cleavage of oxygen molecule O2 takes place, thus one atom of oxygen is incorporated into the substrate molecule, and the second atom of oxygen is released as water.
NADPH + H+ + O2 + RH → NADP+ + H2O + R-OH
Fe3+-P-450-RH + e– (NADPH) → Fe2+-P-450-RH
O2-Fe2+-P-450-RH + e– (NADPH) → Fe3+-P-450 + R-OH + H2O
RH – substrate (xenobiotic)
Enzyme system of cytochrome P450 is huge heme protein family (heme binds O2). It includes approximately 70 isoenzymes that perform not only xenobiotics detoxification, but metabolic processes of endogenous molecules: steroid hormones production, bile acids production, eicosanoid production, unsaturated fatty acids production, etc. Many types of cytochrome P450 are found in the liver, in the adrenal cortex (steroid hormone synthesis) and in other organs.
There are three typical features for this system: wide substrate specificity, inducibility and important gene polymorphism.
In long-term load by particular compound there is in a period of few days induction of synthesis of enzymes of endoplasmic reticulum. Induction of synthesis of particular enzymes that metabolise that particular compound (hence this enzyme is inducible). This leads to faster biotransformation of all substances metabolised by this particular enzyme (i.e. faster biotransformation of even different compounds than that one that caused induction). This is basis of many drug interactions, when administration of particular drug is able to cause change in metabolism of simultaneously administered drugs. Described effect dissipates when inducing substances is removed.
Important gene polymorphism is typical for enzymes of cytochrome P450 system. There are persons with high activity of particular enzyme, therefore they are able to metabolise particular substances quickly (quick metabolisers) and at the same time there are persons with low activity of the same enzyme – slow metabolizers.
Next enzymes that are important in biotransformation:
1) Catalase – it catalyses alcohol and several amines oxidation; its substrate is H2O2.
2) Dehydrogenases:
a) alcohol dehydrogenase (ADH) is localised in hepatocyte cytoplasm. This enzyme catalyses oxidation of alcohols to aldehydes. ADH is inducible enzyme – it is induced by ethanol.
b) aldehyde dehydrogenase (ALDH) is localised in hepatocyte mitochondria.
Result of the first phase
First phase may have these results:
1) increased polarity of xenobiotic
2) inactivation of xenobiotic (detoxification)
3) bioactivation of xenobiotic (pharmaceuticals X carcinogens) – there is potential danger of damage
Bioactivation of xenobiotic
Some carcinogens require for their toxic effect preceding activation. Then they are denoted as procarcinogens (maternal carcinogens), these are converted in the body to final carcinogens. This conversion is catalysed by enzymes of the first phase. Produced carcinogens are covalently bound to some biological macromolecule – DNA, protein, phospholipid. Examples of indirect carcinogens are as follows: aflatoxin B1 and benzo(a)pyrene in their biotransformation highly reactive epoxides are produced. Epoxides are either metabolised by the enzyme epoxide hydrolase to diols – thus deactivated, or they react with bases of nucleic acids – thus bind to DNA and produce mutagenic effect.
Second (conjugation, synthetic) phase – conjugation
Conjugation is process when xenobiotic is bound to high polar endogenous compounds (this is however not absolutely true – e.g. binding of methyl). Conjugation may undergo either original substance (in case that it contains suitable function groups and therefore it was not necessary to proceed through first phase), or more commonly product of first phase of biotransformation (i.e. suitable function groups were added in the first phase). Both enzymes (transferases), and conjugation agents (endogenous substance that must be in active form – e.g. UDP-glucuronate) are required for conjugation processes. Products of these processes are called conjugates.
Organism utilizes these conjugation agents:
1) Glucuronic acid (UDP-glucuronate, glucuronidation, UDP-glucuronyltransferase)
2) Sulphate (PAPS = phosphoadenosine phosphosulfate – active sulphate, esterification)
3) Acetate (acetyl-CoA, acetyltransferases)
4) Cysteine (glutathione, glutathione-S-transferases)
5) -CH3 (SAM = S-adenosyl methionine, methyltransferase)
6) Glycine, glutamine (amidation)
1) Glucuronic acid
Glucuronic acid is the most common conjugation agent. It is necessary to activate it by UTP, thus UDP-glucuronate is produced. Only this form is able to react with xenobiotic molecule – conjugation is catalysed by the enzyme UDP-glucuronyltransferase. UDP-glucuronate is bound through atom of oxygen (O-glucuronides) or through atom of nitrogen (N-glucuronides). In addition bilirubin or steroids (i.e. endogenous substances) are also excreted as glucuronic acid conjugates. More details in Subchapter 6/4.
2) Sulphate conjugation
Sulphate conjugation is the second most important conjugation reaction. Sulphate is donated by active sulphate (that is 3´-phosphoadenosine-5´-phosphosulfate (PAPS)). Reaction is catalysed by cytosolic enzyme sulfotransferase. Sulphate conjugates may be cleaved by sulfatases. Endogenous substances sulphation is important in estrogen metabolism.
3) Cysteine (glutathione)
Cysteine is donated by tripeptide glutathione (γ-Glu-Cys-Gly, GSH). Conjugation of GSH with changed xenobiotic molecule is catalysed by glutathione-S-transferases:
R+GSH → R-S-G R-S-G
Glutathione conjugate is changed before its excretion. At first glutamate and glycine are cleaved off and remaining cysteine is acetylated by acetyl-CoA, thus producing mercapturic acids (that is conjugates bound through S with N-acetylcysteine).
Conjugation significantly increases hydrophilic character of xenobiotics. This worsens their ability to pass across membranes, thus facilitating their excretion (by the liver to bile, or by the kidneys to urine). In large intestine are some conjugates cleaved by bacteria (O-glucuronides by the enzyme β-glucuronidase). This process increase their lipophilic character. Xenobiotic are thus reabsorbed to the body (enterohepatic circulation), therefore their excretion is worsened and the compound is retained in the body
Metabolism of selected xenobiotics
In the following summary is described metabolism of selected xenobiotics – ethanol, methanol, ethylene glycol, toluene, and acetylsalicylic acid.
Ethanol
Ethanol is absorbed in whole digestive system (even in mouth and stomach), most of it is however absorbed in the small intestine. After absorption it is equivalently distributed in all tissues and body fluids. Its levels have highest peak after 60-90 minutes (velocity of absorption however depends on several factors). 90 % of absorbed ethanol is metabolised, 10 % is unchanged eliminated in urine, breath and sweat. Ethanol is eliminated by zero order kinetics (more detail in Subchapter 2/4). This elimination has velocity approximately 1 g / 10 kg of body weight per hour. This means that concentration decreases 0,15 ‰ per 1 hour. Lethal dose is approximately 6-8 g of ethanol in 1 kg of body weight, however inter individual difference could be great. Interestingly ethanol decreases antidiuretic hormone secretion.
Metabolism of ethanol
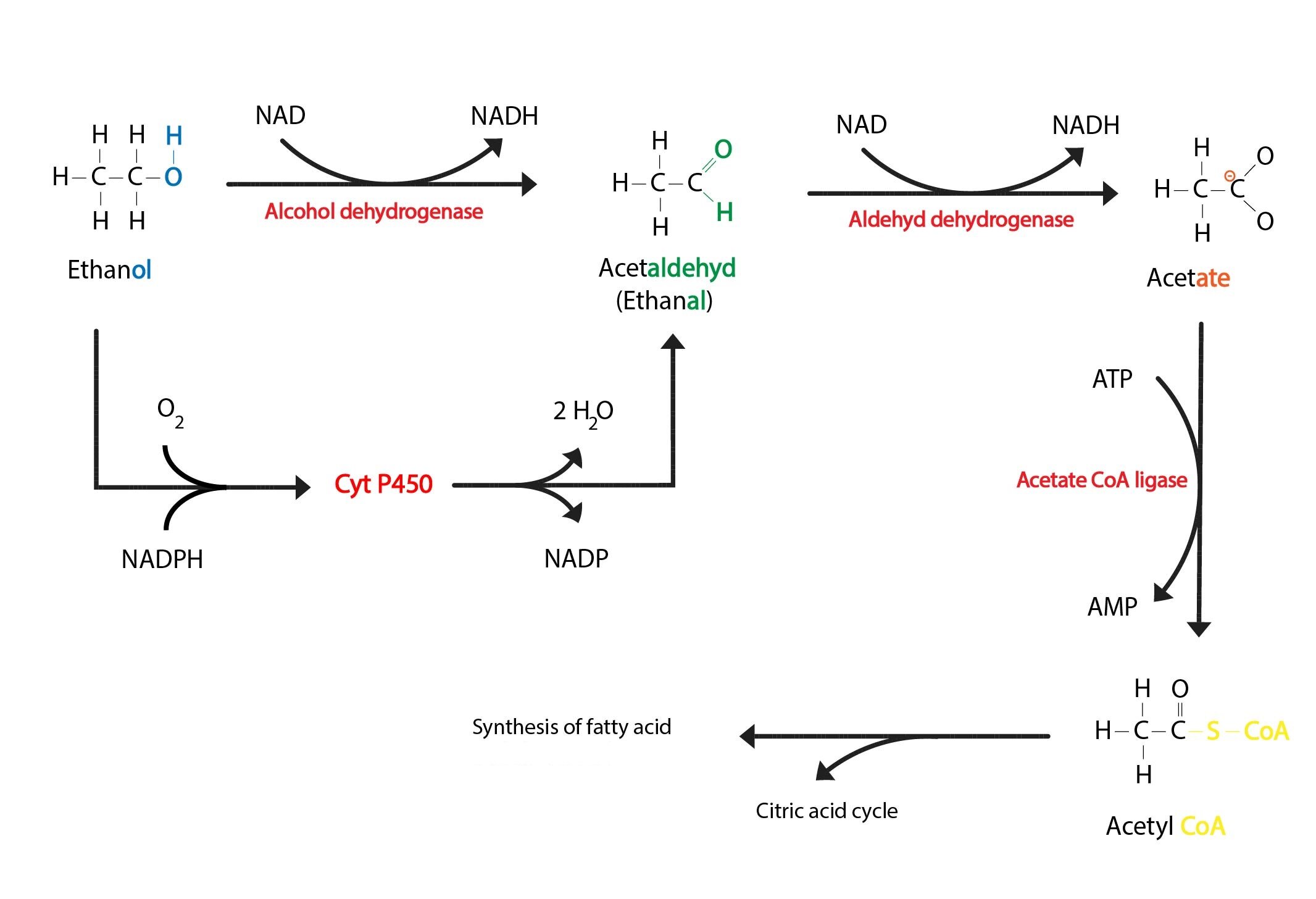
Metabolism of ethanol is mainly located in the liver. In hepatocyte cytosol oxidation of ethanol to acetaldehyde (ethanal) takes place, it is catalysed by the enzyme alcohol dehydrogenase (ADH). This reaction is rate limiting (it depends on availability of NAD+). In mitochondrial matrix is then acetaldehyde oxidised to acetate, this step is catalysed by the enzyme aldehyde dehydrogenase. Acetate is converted to acetyl-CoA by the enzyme acetate-CoA-ligase (ATP dependent reaction). Acetyl-CoA is then degraded in the TCA cycle or it is used in synthesis of fatty acids or cholesterol.
NADH is reoxidised in the ETC. Its capacity is however limited, thus in excess of ethanol NADH cumulates, therefore gluconeogenesis and β-oxidation are inhibited and the TCA cycle is slowed. These are consequences of both lack of oxidised cofactors (NAD+), and shift in some reactions equilibria – e.g.:
Malate ↔ oxaloacetate
Lactate ↔ pyruvate
Thus risk of hypoglycaemia development is increased (in case that saccharides are administered insufficiently), also lactate acidosis (lactate accumulates), hyperuricemia (increased lactate in the bloodstream decreases capacity of the kidney to excrete uric acid), and TAG accumulation in the liver may develop.
Acetaldehyde cumulates in the blood stream. It is reactive substance – adducts with proteins and nucleic acids are produced. Acetaldehyde also causes nausea and vomiting.
Apart from above mentioned enzymes is ethanol metabolised by catalase and by cytochrome P450 system (it is so called MEOS = microsomal ethanol oxidising system, mainly CYP2E1). MEOS catalyses the same reaction as alcohol dehydrogenase (i.e. conversion of ethanol to acetaldehyde), mechanism is however quite different:
CH3CH2OH + O2 + NADPH → CH3CHO + NADP+ + 2 H2O
When alcohol dehydrogenase is saturated (respectively when NAD+ is depleted) MEOS comes into action. Its advantage is its inducibility. In case that ethanol consumption is increased MEOS is induced, smooth endoplasmic reticulum is multiplied thus accelerating alcohol metabolism. Therefore metabolism of many other compounds is accelerated (drugs metabolism is quite different in chronic alcoholism). Disadvantage of metabolism by MEOS is increased production of reactive oxygen species. ROS cause damage of hepatocytes.
Caloric contents of ethanol is 29,4 kJ/g. Alcoholic beverages provide significant amounts of energy.
Long-term excessive consumption of alcohol causes damage of many organs (liver, cardiovascular system, CNS). Safe (or even beneficial) consumption limit is individual, generally is however considered within 20-40 g of alcohol per day for men and 20-30 g for women. 20 g of ethanol is approximately in 200 ml of wine, 0,5 l of beer and 50 ml of 40% spirit.
For you to get the picture of ethanol contents in alcoholic beverages (ethanol is given in volume % (v/v)) – bottle of beer (0,5 l with 4 % v/v of alcohol) contains 20 ml = 16 g of ethanol, bottle of wine (0,7 l with 12 % v/v of alcohol) contains 84 ml = 66 g of ethanol.
Methanol
Methanol is particularly dangerous poison. It is dangerous because it may be easily confused with ethanol (by taste or smell pure methanol almost pasts all recognition from ethanol). Methanol has weaker narcotic effect than ethanol, however its elimination is slower (slow oxidation) thus prolongs period of drunkenness.
Metabolism of methanol
Methanol is metabolised by the same enzymes as ethanol (alcohol dehydrogenase and aldehyddehydrogenase). This fact is used in treatment of poisoning. In metabolism formaldehyde and formic acid are produced:
CH3OH → HCHO → HCOOH
Methanol poisoning causes more severe nausea and vomiting than ethanol poisoning (because formaldehyde is produced). Simultaneously breath discomfort and convulsion develop. After drunkenness episode latent period occurs (12-18 hours), after latent period develop headache, back pain, irreversible vision damage (ganglion cells in retina are damaged – destructive inflammation leads to atrophy, this inflammation is caused by formaldehyde), fast, shallow breathing, increased heart rate, decreased blood pressure and cyanosis. Methanol toxicity is in addition based even on formic acid accumulation – i.e. metabolic acidosis develops.
Principle of treatment is ethanol administration. Ethanol competes with methanol for enzymes. Patient is thus kept few days in drunkenness by ethanol (concentration of ethanol approximately 1 ‰). Simultaneous disturbances of internal environment.
For you to get the picture some doses causing severe damages are as follows:
Serious poisoning: 5-10 ml
Blindness: 7-15 ml
Lethal dose: ~ 30 ml
Ethylene glycol (HO-CH2CH2-OH)
Ethylene glycol is part of antifreeze substances (Fridex). It is sweet and very poisonous (destroys blood vessels, it is nephrotoxic, it is metabolised to oxalic acid and glycolic acid that both cause metabolic acidosis, etc.). Severe poisoning occurs after consumption of 50 ml of ethylene glycol, lethal dose is 100 ml.
Ethylene glycol metabolism
Ethylene glycol is oxidised – at first aldehydes are produced, then carboxylic acids:
Ethylene glycol → glycolaldehyde → glycolic acid → glyoxylic acid → oxalic acid
Two main end products – glycolic acid and oxalic acid:
HO-CH2-CH2-OH → HO-CH2-COOH → HOOC-COOH
After consumption of ethylene glycol there is latent period with duration of few hours, then vomiting, severe abdominal pain, drunkenness (i.e. effect on central nervous system), consciousness disorders or coma, convulsion, breathing problems develop. After 1-3 days oliguria or even anuria develop. Treatment is similar to methanol poisoning treatment (i.e. ethanol administration).
Toluene
Toluene is organic solvent that may enter body through respiratory tract (i.e. inhalation) or through skin (transdermal) Acute poisoning manifests as drunkenness, nausea and unconsciousness may develop. In some cases fatal coma develops. Long-term abuse causes very serious consequences – irreversible CNS damage.
Metabolism of toluene
At first is toluene oxidised to benzoic acid, that is then conjugated with glycine, thus producing hippuric acid. Hippuric acid is excreted in urine thus it may be assessed – it is prove of toluene exposition.
Acetylsalicylic acid (analgesic, antipyretic, anti-inflammatory drug)
Acetylsalicylic acid is ester of salicylic acid (2-hydroxybenzoic acid). Acetylsalicylic acid is plentifully administered medicament – it is active ingredient of Aspirin. It inhibits cyclooxygenase, enzyme that is crucial for some eicosanoids (thromboxane, prostaglandin) synthesis.
lipoxygenase cyclooxygenase
Leukotrienes ←←←←←←← arachidonic acid →→→→→→→ prostaglandins, thromboxanes
It performs irreversible action (it is covalently bound to enzyme), therefore its effect lasts even after elimination from body (t1/2 = 20 minutes). Duration of effect depends on velocity of new cyclooxygenase molecules synthesis. Usual therapeutic dose is 0.5-1 g, lethal dose is 10-30 g.
Metabolism of acetylsalicylic acid
When ingested acetylsalicylic acid is hydrolysed (in gut and in the blood stream). Thus producing salicylic acid and acetic acid.
Salicylic acid contains suitable polar functional groups (hydroxyl group was “uncovered” by ester bond hydrolysis, this reaction in addition removed steric barrier for access to carboxyl group). In the liver conjugation takes place – carboxylic group is conjugated with glycine, thus producing salicyluric acid (conjugation with glucuronic acid is also possible).
Produced conjugate is excreted in urine (unchanged salicylate is excreted quite slowly).
Acetylsalicylic acid damages gastric mucosa, inhibits platelet aggregation (it prolongs bleeding time), causes bronchoconstriction (PGE2 synthesis is decreased) and causes overproduction of leukotrienes, that may cause asthmatic fit.
Toxic effects: CNS stimulation, then deep depression, increased O2 consumption (thus increased respiratory rate). Death occur because of respiratory failure.
Subchapter Authors: Josef Fontana, Martina Šajdíková and Patrik Maďa